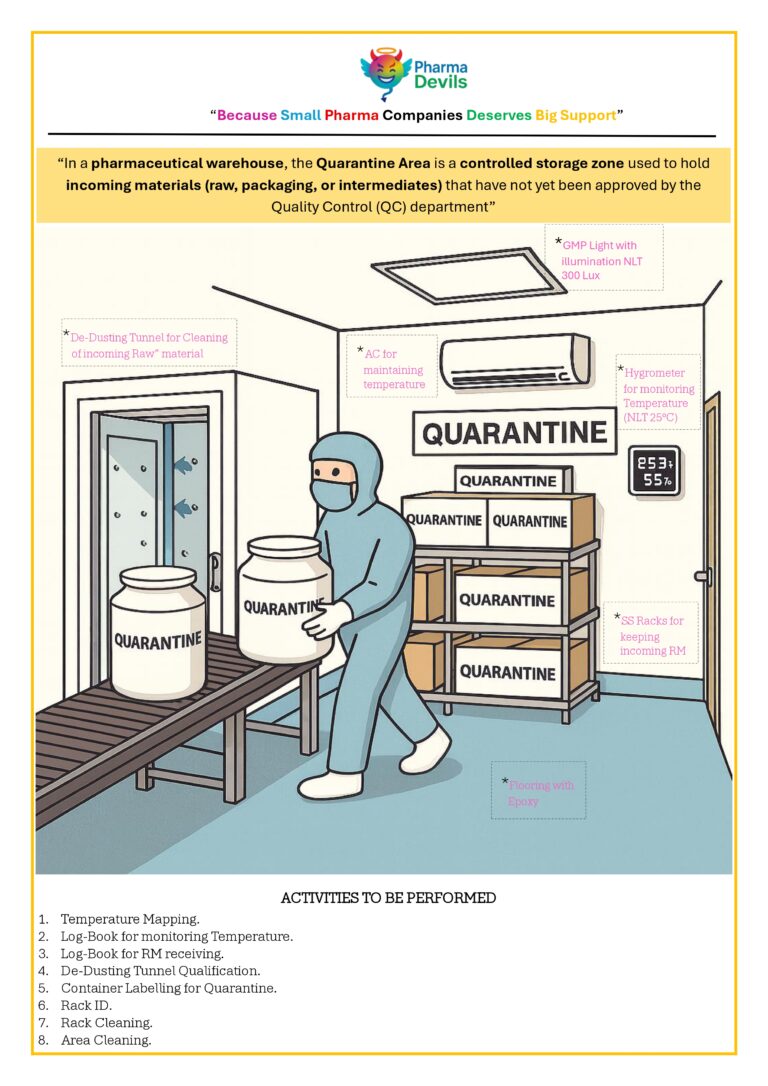
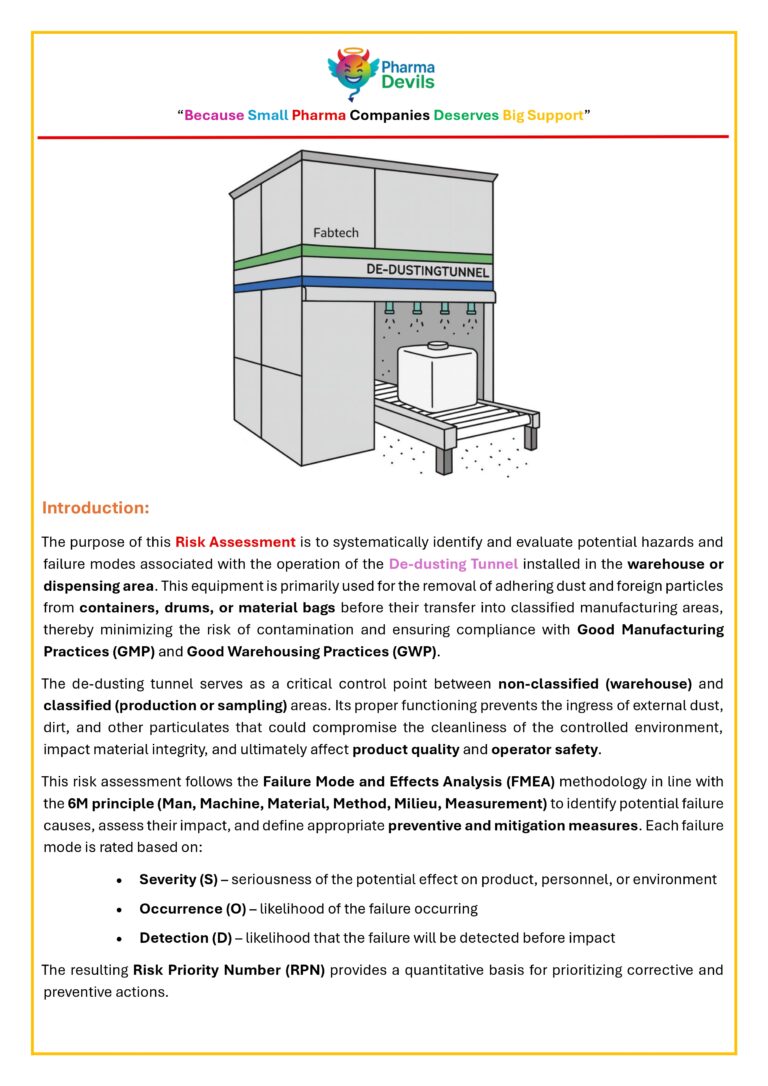
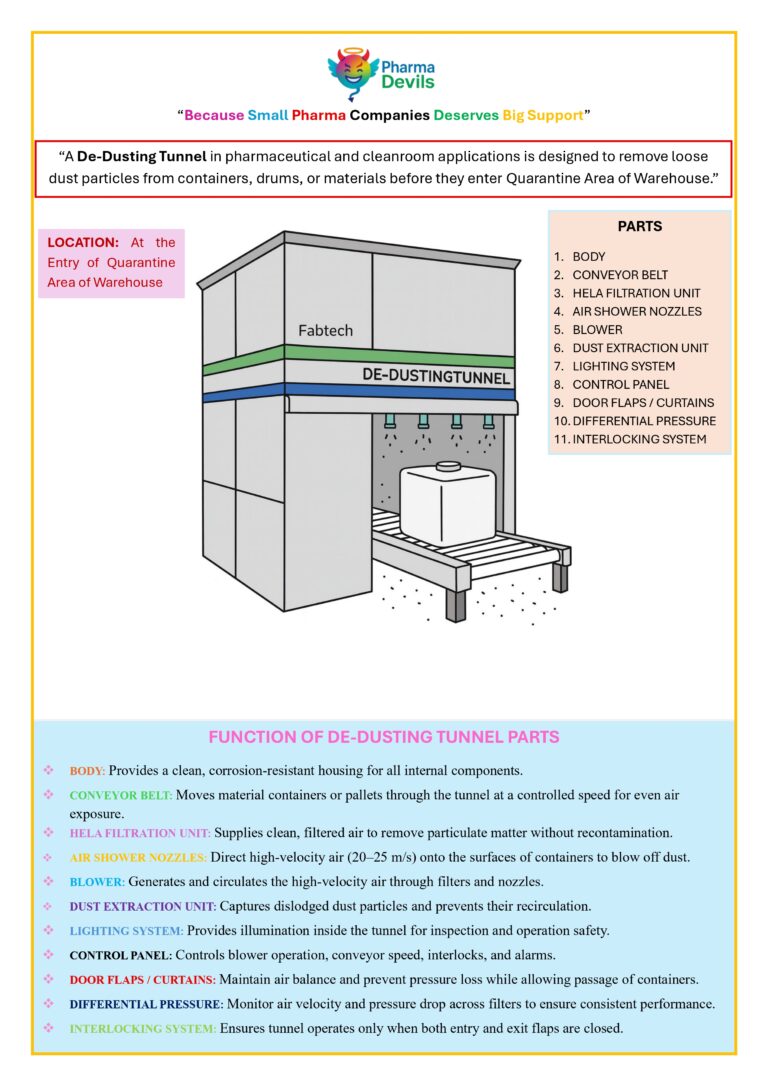
DECODING WAREHOUSE (CHAPTER 4 – STAGING AREA)
Material Receiving Checklist at Staging Area (Before Quarantine) 1. Material Receiving Verification: 2. Visual Inspection of Containers: 3. Identification & Labeling: 4. Segregation & Handling: 5. Documentation & Recordkeeping: 6. Environmental & Safety Checks: 7. Transfer to Quarantine Area: Final…