







Post Views: 135

Post Views: 181

Post Views: 233 A Risk Assessment for QC instruments in the pharmaceutical industry is critical to ensure accurate, reliable, and compliant data. The purpose of

Post Views: 241 In the pharmaceutical industry, “deviation” refers to any departure from approved processes, procedures, specifications, or expected outcomes. These deviations can occur during

Post Views: 404 Change Control in the pharmaceutical industry is a critical part of Quality Management System (QMS). It ensures that all changes to a

Post Views: 280 Pharmaceutical Artwork refers to the design and layout of packaging, labels, and informational leaflets for medicines. It plays a crucial role in

Post Views: 735 Line clearance in the pharmaceutical industry is a critical process that ensures a production line is free from contaminants, previous product residues,
Post Views: 618 Regulatory affairs (RA) in pharma is a critical function that ensures pharmaceutical products meet legal and safety standards before they reach the
Post Views: 408 Supply chain management (SCM) in the pharmaceutical industry is a complex and highly regulated process that ensures the efficient flow of medicines

Post Views: 927 Pharmaceutical policies in a manufacturing plant focus on ensuring product quality, safety, compliance, and efficiency. These policies are governed by regulatory agencies

Post Views: 692 Self-inspection in the pharmaceutical industry is a critical aspect of Good Manufacturing Practices (GMP) compliance. It involves an internal audit conducted by
Post Views: 651 An Environment Monitoring System (EMS) in pharmaceuticals ensures that critical areas comply with the regulatory requirements for cleanliness, temperature, humidity, pressure, and

Post Views: 344

Post Views: 800
Post Views: 487 A Project Validation Plan (PVP) for Quality Control (QC) facilities outlines the structured approach to ensure that the QC facilities comply with

Post Views: 783
Post Views: 396 Pharmaceutical formulation and development involve transforming active pharmaceutical ingredients (API’s) into safe, effective, and patient-friendly medications. This process encompasses several key stages:
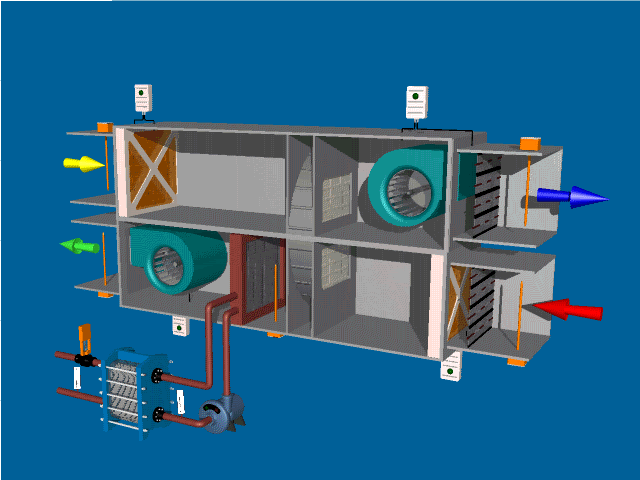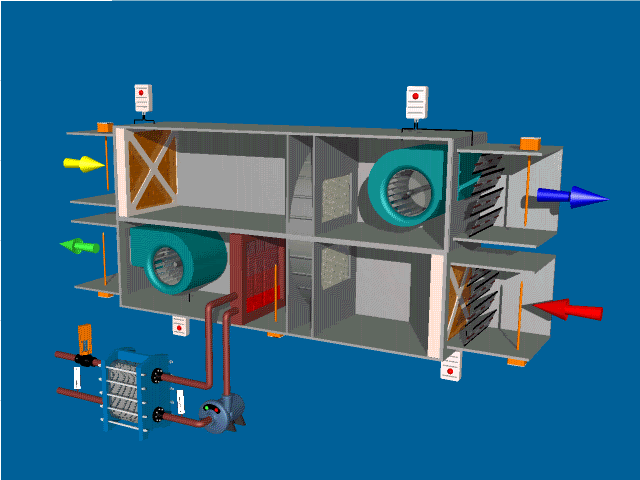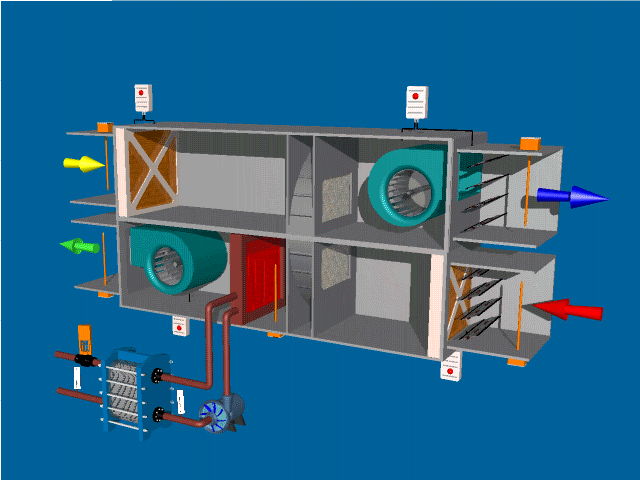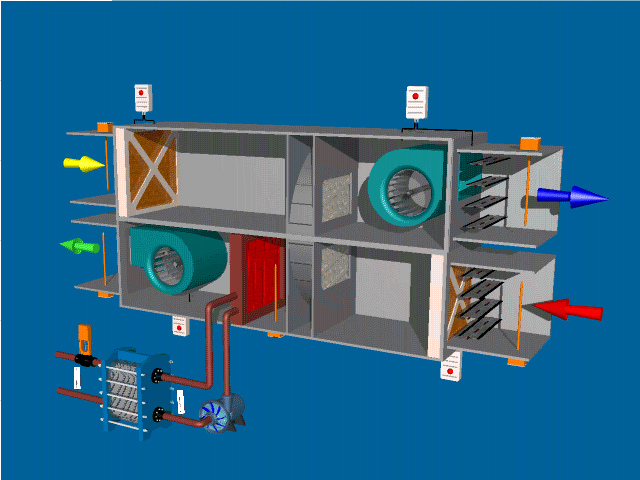
Post Views: 1,643 An Air Handling Unit (AHU) is a central component of HVAC (Heating, Ventilation, and Air Conditioning) systems. Its primary function is to

Post Views: 1,748 A Hormone is defined as a chemical released by a cell or a gland in one part of the body that sends out

Post Views: 2,998 HIRA (Hazard Identification and Risk Assessment) is a structured method for detecting and evaluating hazards in process facilities/systems. HIRA detects potential risks that
Post Views: 4,951 Preventive Maintenance is performed when the equipment is in working condition and satisfactorily produces a desired output within the capacity. Preventive Maintenance in
Post Views: 2,538 A User Requirement Specification (URS) is a formal document that outlines the needs of a user for a system, product, or service. In the

Post Views: 2,173 The URS is a definition of requirements to fulfil the demands of the process from the Users’ point of view. The success of
Post Views: 1,585 Oncology in the pharma industry is the development and manufacturing of cancer treatments. The term “Oncology” comes from the words “Onco” which means bulk,
Post Views: 2,481 A User Requirement Specification for DPI is a formal document that defines the requirements for using a system in a regulated environment, such
Post Views: 2,918 A User Requirement Specification Ointments is a document that defines the procurement requirements for equipment. It’s usually written early in the validation process,
Post Views: 2,271 A placebo is a “physiologically inert substance or sham intervention (psychological, physical or mechanical) which produces beneficial effects independent of any direct therapeutic effects”. The

Post Views: 4,261 In the pharmaceutical industry, Occupational Exposure Limit (OEL) refers to the maximum acceptable concentration of a hazardous substance in workplace air for

Post Views: 4,190 Lyophilizer Qualification in Pharma is an important aspect in pharma. Lyophilization, also known as freeze-drying, is a process used to preserve a
Post Views: 6,694 Analytical method validation in Pharma is a critical process in the development and implementation of methods used for data analysis in various
Post Views: 21,005 Microbiology SOP in Pharma plays important role in pharmaceuticals. Microbiology is the study of microscopic living organisms, such as bacteria and fungi.

There is no excerpt because this is a protected post.

Post Views: 4,208 A walk-in stability chamber is a controlled environment designed to simulate specific conditions such as temperature, humidity, and light to test the

Post Views: 2,832

Post Views: 2,708

Post Views: 8,145 In general, large companies with multiple sites have one site that serves as “headquarters” for the corporation. It’s the place where (irrespective
Post Views: 4,252
Post Views: 4,016 The User Requirements Specification describes the business needs for what users require from the system. User Requirements Specifications are written early in the

Post Views: 4,571 Stability testing is utilized to determine if the quality of a drug substance or drug product is altered over time by various environmental
Post Views: 5,864 A Critical Process Parameter (CPP) is a term used in pharmaceutical production for process variables which have an impact on a critical

Post Views: 3,403 Every activity in Pharma have some frequencies whether it is revision of documents, qualification, validation, stability, Re-testing etc. While Acceptance criteria means numerical
Post Views: 6,554 A “media fill” (sometimes known as a “process simulation”) is the performance of an aseptic manufacturing procedure using a sterile microbiological growth medium

Post Views: 3,307 Miscellaneous Documents in Pharma are those documents which does not comes under any dosage form and can be applied in general.
Post Views: 6,130 Pharmaceutical formulation is the process of combining various chemical substances with the active drug to form a final medicinal product, which is

Post Views: 5,220
Post Views: 5,357 All criticalities emerged during the FAT exercise are then checked again at the final site, after installation and verification; additional test cases may
Post Views: 5,061 Microbiology Instruments Qualification plays important role in pharmaceuticals. Microbiology equipment is a large category covering all kinds of items used in microbiology
Post Views: 5,944 An “API Starting Material” is a raw material, intermediate, or an API that is used in the production of an API and

Post Views: 5,954 Dry powders for oral suspension are powder mixtures that require the addition of water (reconstitution) at the time of dispensing and are mostly

Post Views: 4,272 Pharmaceutical Aerosols are pressurized dosage forms containing one or more active ingredients which upon activation emit a fine dispersion of liquid and/or
Post Views: 5,452 In the world of Pharmaceuticals, a Factory Acceptance Test (FAT) is simply a series of test for a newly manufactured equipment. With a Factory
Post Views: 4,981 The User Requirement Specification for Microbiology (URS) is offered to help the user with the crucial facets of fabrication, facilities for installing

Post Views: 2 During Hold Time Study in Pharma, Good manufacturing practices (GMP) require that arrangements should be made to ensure that the dispensed raw
Post Views: 8,654 The pharmaceutical industry places a lot of importance on the temperature mapping study of cold chambers, incubators, warehouses, transport validation, stability chambers,
Post Views: 6,695 In the domain of microbiology, the process by which it is proven by laboratory tests that a method’s performance characteristics satisfy the

Post Views: 5,567 By evaluating the tests for weight variation, hardness, and friability, for example, IPQA Instruments in Pharma assess the test results from in-process

Post Views: 44,142 Nitrogen System Qualification plays important role in pharmaceuticals. PSA (Pressure Swing Adsorption) Based Nitrogen Plant is to produce Nitrogen gas from Atmospheric

Post Views: 9,497 Compressed air Qualification in pharma is proof that certain factors, including aerosol particle content, dew point, liquid water concentration, vapour content, oil
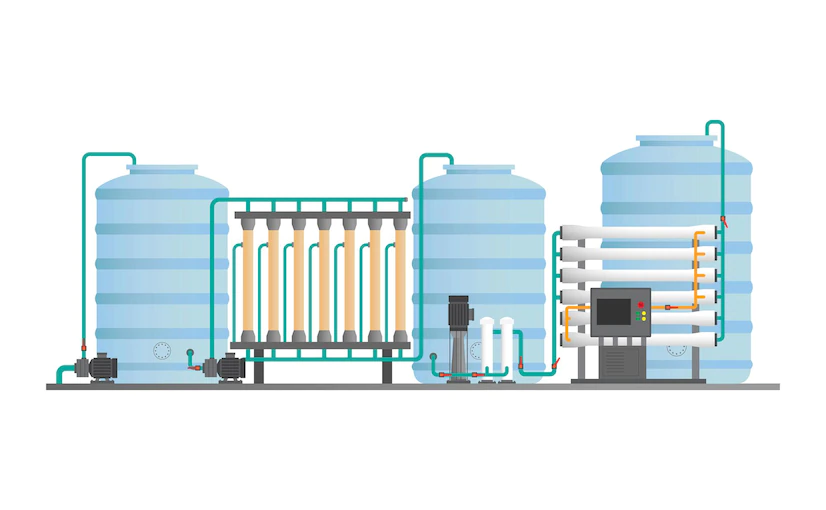
Post Views: 18,904 “Water” forms the most important ingredient in the pharmaceutical manufacturing process and also finds a major use in cleaning of equipment before
Post Views: 9,842 The User Requirements Specification (URS) is offered to help the user navigate the crucial aspects of fabrication, facility for installation of required
Post Views: 53,126 Manufacturing SOP in pharma, a component of the pharmaceutical business, is the process of synthesizing pharmaceutical medications on an industrial scale. A
Post Views: 31,413 Engineering SOP in Pharma is concentrated on planning, constructing, and enhancing pharmaceutical manufacturing facilities. Despite the fact that certain Pharma engineers also

Post Views: 4,147 The ISO 9001 standard for Quality Management Systems first called for manuals in pharma as part of its requirements. Top-level publications that

Post Views: 11,994 The HR SOP in Pharma (HR department) of an organisation manages human resources and handles a variety of employment-related tasks, including hiring,

Post Views: 5,234 One of the most significant elements of the entire medication production process is quality assurance in pharma. It will not only enable

Post Views: 58,616 Cleaning Validation goal is to guarantee that cleaning procedures are effective in removing product residue, cleaning agent residue, and live microorganisms from
Post Views: 46,830 In the pharmaceutical sector, CSV is crucial for enhancing product quality, improving process performance, and supporting high-quality goods. The primary advantage of

Post Views: 5,720 Pharmaceuticals must include quality control SOP as a crucial component. The World Health Organization (WHO) defines quality control (QC) as the total

Post Views: 1,021 When raw materials (Active & Excipients) and packing materials are received and stored under the necessary storage conditions, this is known as
Post Views: 26,449 Information technology SOP in Pharma are used in the pharmaceutical industry to generate, process, store, retrieve, and communicate many types of data

Post Views: 4,634 Utility in Pharma like WFI, RODI, Compressed Air, Nitrogen, Air Handling Units (AHU) and HVAC (Heating, Ventilation and Air Conditioning) systems support

Post Views: 85,345 In the pharmaceutical industry, quality risk assessment in pharma entails the identification of hazards as well as the investigation and assessment of

Post Views: 11,446 Process validation, according to the FDA, is “…the gathering and evaluation of data, from the stage of process design to commercial production,
Post Views: 9,028 Every workplace has a specific set of hazards, and safety SOP in Pharma can help. The pharmaceutical manufacturing sector entails a variety
Post Views: 3,996 Large volume parenteral equipments qualifications in pharma play a significant role. Injectable aqueous medication products that have been terminally sterilized (autoclaved) and
Post Views: 4,082 Form Fill Seal Equipments qualifications in Pharma are crucial. To prepare sterile items, form fill seal (FFS) technology uses automated computer control.
Post Views: 4,004 Ampoule Equipments Qualification plays important role in pharma. An ampoule, also known as an ampul or an ampule, is a tiny sealed
Post Views: 2,960 Opthalmics Equipments Qualifications are crucial in the pharmaceutical industry. Opthalmics (Eye drops) are liquid drops that are often applied in small volumes,

Post Views: 4,809 Dry Powder Injections Equipments Qualifications in pharma play a significant part in dry powder injection equipment. Dry powder injections are solid materials
Post Views: 6,878 Oral liquids in Qualifications in Pharma are crucial. Pharmaceuticals include syrup, oral suspension, oral solution, oral drop, oral emulsion, mixture, linctus, and
Post Views: 5,365 Ointments qualifications in pharma plays a significant role. Ointments are cosmetic products that are applied externally to the skin. When applied to

Post Views: 3,348 Softgel Capsules Qualifications in Pharma plays important role in pharma. A Softgel Capsules have an exterior shell made of solid material that

Post Views: 4,593 Hardgel Capsules Qualifications plays important role in pharma. Hardgel Capsules are solid dose forms that contain one or more active ingredients and/or

Post Views: 8,256 Tablets Qualifications in Pharma plays important in pharma. Tablet is an oral solid dose form for medications (OSD). Tablets are a solid