RISK BASED INSPECTION (SCHEDULE M-POINT 1.6)

RISK BASED INSPECTION (SCHEDULE M-POINT 1.5)

RISK BASED INSPECTION (SCHEDULE M-POINT 1.4)

RISK BASED INSPECTION (SCHEDULE M-POINT 1.3)

RISK BASED INSPECTION (SCHEDULE M-POINT 1.2)

RISK BASED INSPECTION (SCHEDULE M-POINT 1.1)

HORMONES IN PHARMA

A Hormone is defined as a chemical released by a cell or a gland in one part of the body that sends out messages that affect cells in other parts of the organism. Only a small amount of hormone is required to alter cell metabolism. Hormonal preparations are one of the major classes of drugs used […]
USER REQUIREMENT SPECIFICATION FOR OPHTHALMIC
A User Requirement Specification (URS) is a formal document that outlines the needs of a user for a system, product, or service. In the pharmaceutical industry, a URS is a crucial document that ensures that equipment, processes, or systems meet regulatory standards and user needs.
USER REQUIREMENT SPECIFICATION FOR AMPOULES

The URS is a definition of requirements to fulfil the demands of the process from the Users’ point of view. The success of a GMP clean room design is dependent on a clear and concise User Requirement Specification (URS) to take through a successful Design Qualification (DQ).
ONCOLOGY IN PHARMA
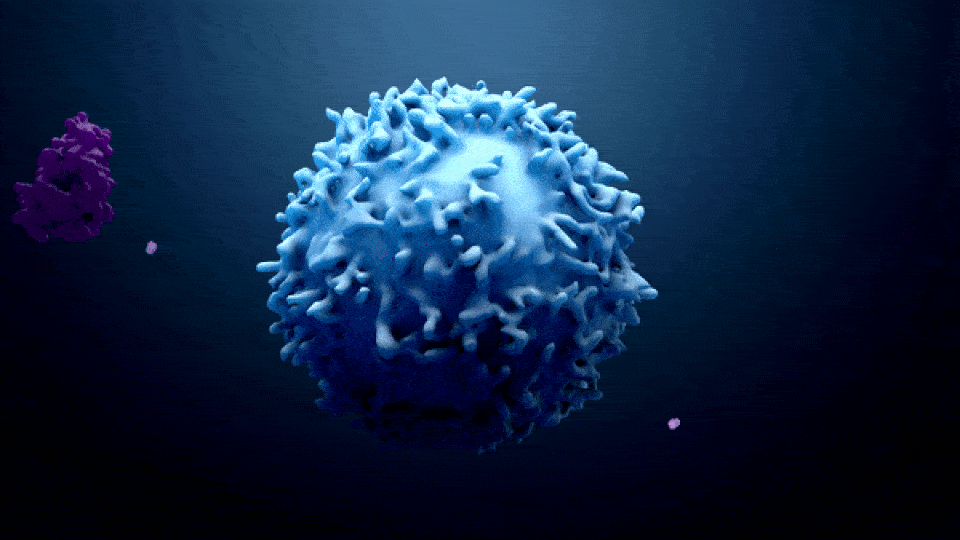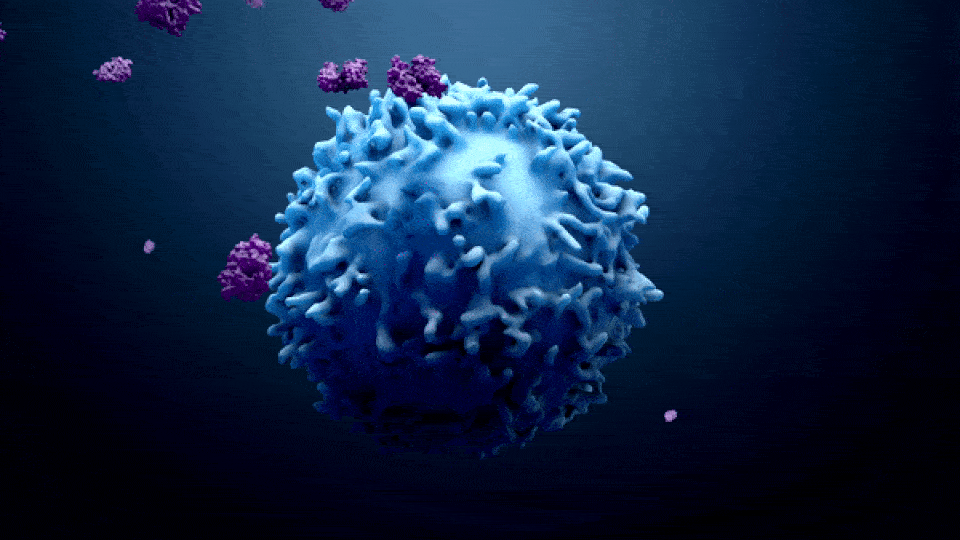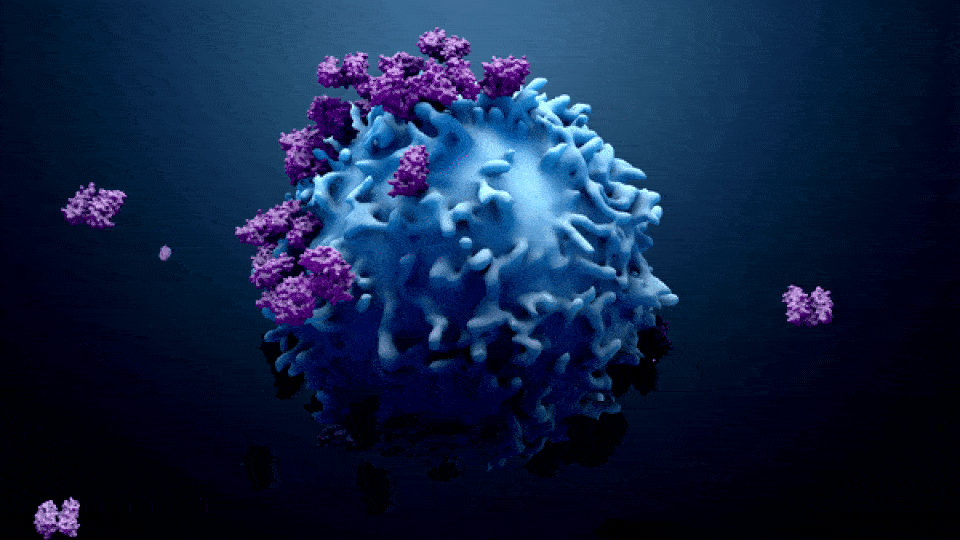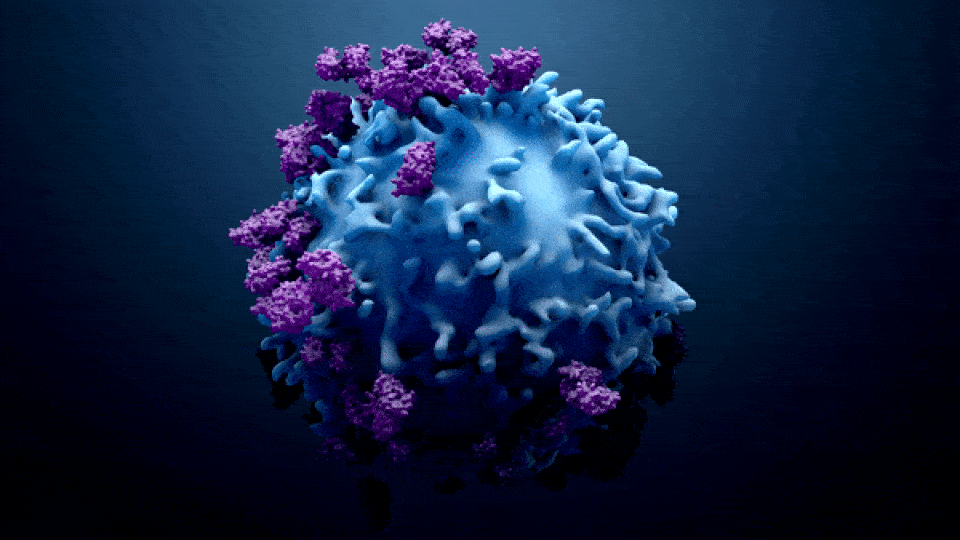
Oncology in the pharma industry is the development and manufacturing of cancer treatments. The term “Oncology” comes from the words “Onco” which means bulk, mass, or Tumor, and “Logy” which means study. Oncology is a branch of medicine that specializes in the diagnosis and treatment of cancer, including preventative medicine and medical oncology. Medical oncology uses drugs such as […]






