NITROGEN SYSTEM QUALIFICATION

Nitrogen System Qualification plays important role in pharmaceuticals. PSA (Pressure Swing Adsorption) Based Nitrogen Plant is to produce Nitrogen gas from Atmospheric compressed air. Air passes through Carbon Molecular Sieves (CMS) at a certain pressure, the moisture, Oxygen, and CO2 are selectively adsorbed, and balance nitrogen comes out and collects in the receiver. Compressed air […]
COMPRESSED AIR QUALIFICATION IN PHARMA

Compressed air Qualification in pharma is proof that certain factors, including aerosol particle content, dew point, liquid water concentration, vapour content, oil aerosols, and other contaminants, are below acceptable limits and do not contaminate the product. In order to deliver high-quality compressed air at the outlet, air compressor units have an air unit system. It […]
WATER SYSTEM QUALIFICATION IN PHARMA
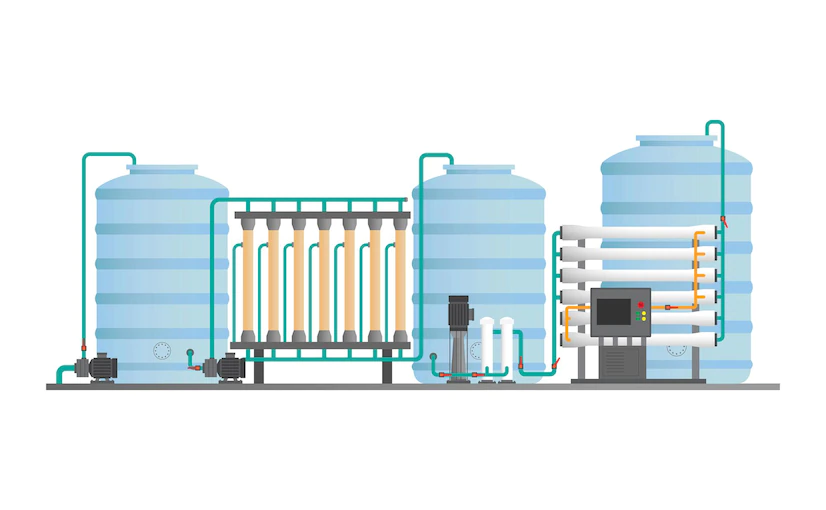
“Water” forms the most important ingredient in the pharmaceutical manufacturing process and also finds a major use in cleaning of equipment before and after processing. However due to its tendency to give way to microbiological proliferation during storage and distribution, it becomes a “critical” ingredient as well. Thus emphasis is given on water system maintenance […]
UTILITY IN PHARMA

Utility in Pharma like WFI, RODI, Compressed Air, Nitrogen, Air Handling Units (AHU) and HVAC (Heating, Ventilation and Air Conditioning) systems support the manufacturing process. Quantitative and qualitative requirements: In order to be deemed satisfactory, utility in pharma must meet a number of qualitative and quantitative requirements. As a result of input from key departments […]






