Risk Assessment for Black Particles observed in Ampoules
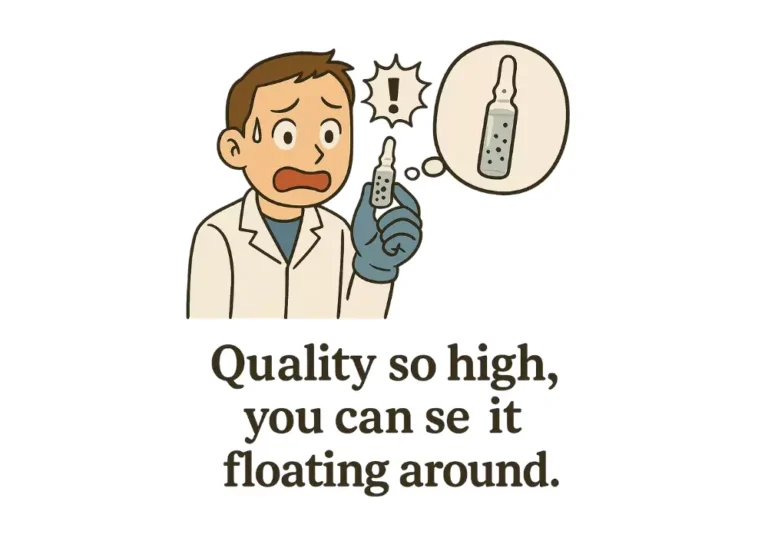
The presence of black particles in ampoules is a serious quality issue in parenteral manufacturing. These particles can originate from various sources during the production, filling, sealing, or storage of ampoules. Risk Assessment for Black Particles observed in Ampoules shall…