

Swab sampling recovery is the study that proves your swab method can reliably remove and detect residues from equipment surfaces during cleaning validation. It quantifies how much of a known residue load applied on a defined area can be recovered, extracted, and measured by your analytical method—reported as % recovery. This factor supports accurate result interpretation and, where justified, correction of swab results.
Purpose
- Demonstrate suitability of swab material, wetting solvent, swabbing technique, and extraction conditions.
- Confirm the analytical method can detect residues at or below the MACO/limit from real surfaces (not only in solution).
- Establish a scientifically justified recovery factor for each product/surface/solvent combination (as applicable).
Typical study design
- Select surfaces representing the equipment: SS316L (common), glass, PTFE, silicone, gaskets, or coated surfaces. Include “worst-case” roughness if relevant.
- Define test area (commonly 25 cm², 10×10 cm, or actual template area used in routine sampling).
- Spike known amount of target residue (API, marker, detergent) onto the area using a calibrated pipette. Choose levels around the acceptance limit (e.g., 50%, 100%, 150% of limit) and include low-level points near LOQ.
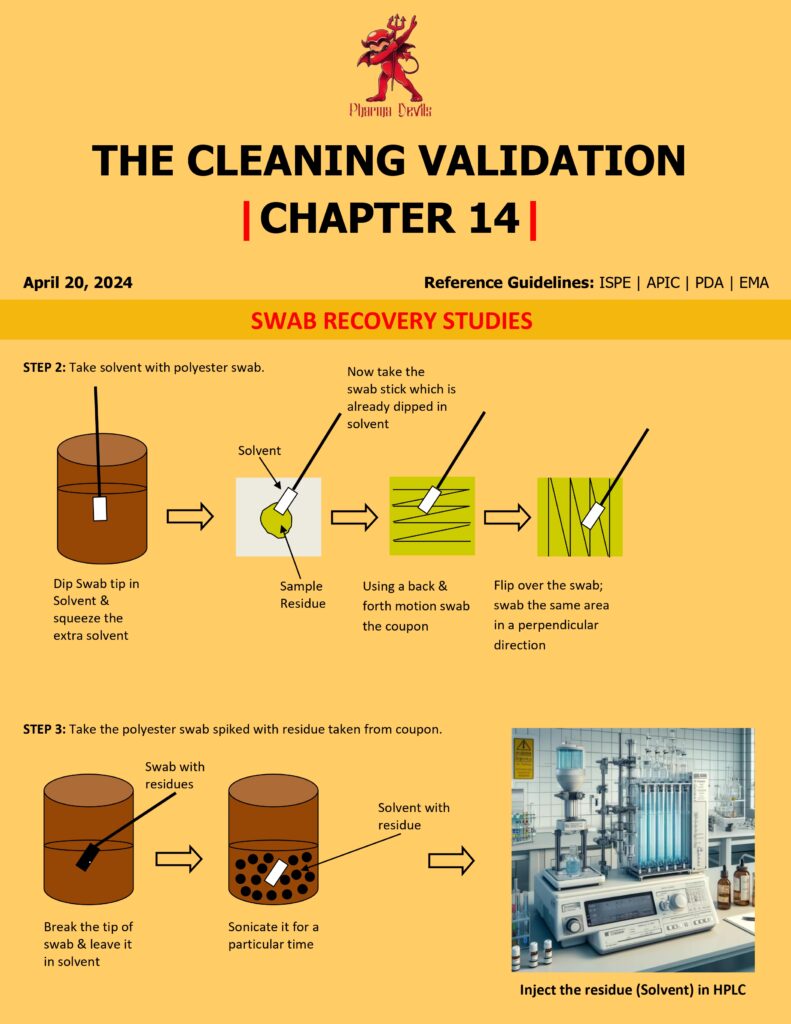
- Dry/age the residue under controlled conditions (time and temperature) to simulate real equipment hold times.
- Swab with controlled technique: pre-wet swab (specified solvent and volume), swab in horizontal + vertical + diagonal strokes, rotate swab head, maintain consistent pressure, and avoid re-contamination.
- Extract the swab in a defined volume of solvent using a validated approach (vortex/sonication/shaking) for a fixed time.
- Analyze extract using the validated method (HPLC/UV/TOC/conductivity, etc.).
- Calculate recovery:
% Recovery = (Measured amount recovered ÷ Spiked amount) × 100
Acceptance and interpretation
Many firms target ≥70% average recovery with acceptable precision (e.g., %RSD). Lower recoveries may be acceptable if consistent, justified, and used with a correction factor. Recovery can vary by surface type, residue chemistry, drying time, and solvent; therefore document worst-case conditions, number of replicates (often n=3–6 per level), and robustness checks.
Key controls
Include blank swabs, surface blanks, solvent blanks, and spiked-swab controls to separate swab extraction losses from surface removal losses.





