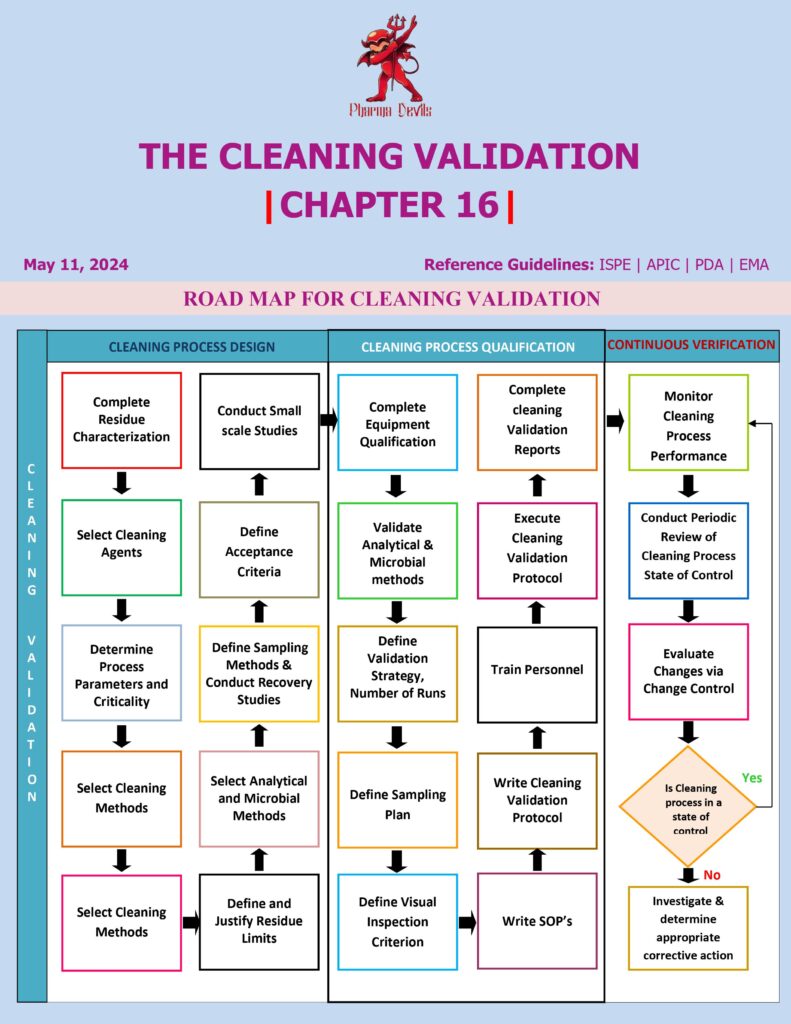
A cleaning validation roadmap is the stepwise plan used to prove equipment cleaning consistently meets predefined acceptance criteria and stays in control throughout routine production.
1) Define scope and build the equipment matrix
List all equipment trains and product-contact parts. Group equipment by design and cleaning method (manual/CIP/COP). Create a matrix linking products ↔ equipment ↔ cleaning procedures so you know which combinations require validation.
2) Risk assessment and worst-case selection
Use a documented risk tool (FMEA or similar) to pick worst-case products and conditions: lowest solubility, highest potency/toxicity (HBEL/PDE), highest batch size, strongest staining, difficult formulation (sticky/oily), and longest dirty hold time. Identify hardest-to-clean locations: spray shadows, gaskets, valves, dead legs, transfer lines, filters, and low-point drains.
3) Develop/optimize the cleaning procedure
Define the cleaning steps and critical parameters: detergent type/concentration, temperature, time, flow/turbulence, mechanical action, rinse quality, and drainability. Establish dirty hold and clean hold times with supporting data.
4) Analytical methods and sampling strategy
Select methods with LOQ below the required limits (HPLC/UV/TOC/conductivity). Decide swab vs rinse (or both). Define sampling locations based on risk and geometry. Perform swab recovery studies and train operators to standardize technique.
5) Set acceptance limits
Calculate limits using HBEL/PDE-based MACO where available. Convert to surface limits (µg/cm²) using shared surface area, batch size, and maximum daily dose. Add limits for cleaning agents, and set microbial/endotoxin criteria if the process warrants it. Define “visually clean” and, if used, establish a Visible Residue Limit (VRL).
6) Write protocols and execute PQ
Prepare approved protocols with clear responsibilities, sampling plan, hold times, and deviation handling. Run PQ—commonly three consecutive successful cleaning runs for each worst-case scenario under routine conditions. Record all critical parameters and verify results meet limits.
7) Closeout and ongoing verification
Issue validation reports, finalize SOPs, and implement continued verification: periodic sampling (as justified), trending, visual inspection, review of CIP cycle data, preventive maintenance, calibration, and change control. Define revalidation triggers (new product, new detergent, equipment change, limit change, repeated deviations). This roadmap ensures both initial proof and sustained cleaning control.





