
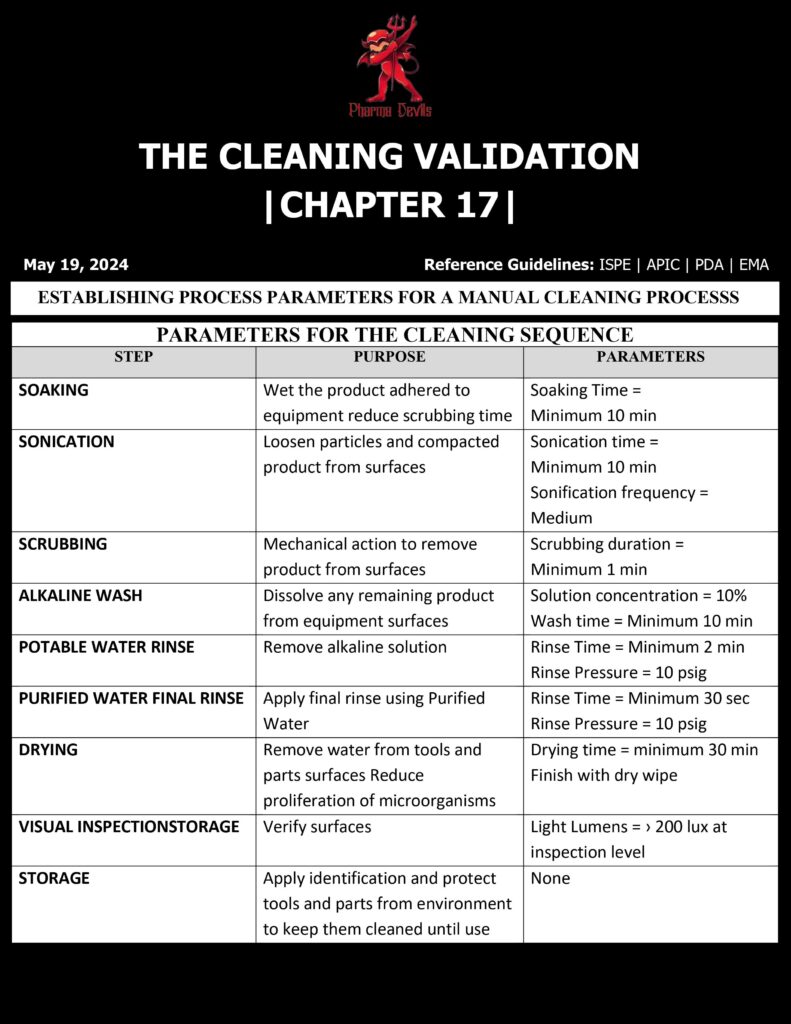
Establishing process parameters for a manual cleaning process in cleaning validation means defining, controlling, and justifying the key variables that ensure consistent removal of product residues and cleaning agents from equipment. Because manual cleaning depends heavily on operator technique, parameters must be clear, measurable, and repeatable, with training and supervision built into the strategy.
1) Define the cleaning sequence and boundaries
Document the stepwise flow: pre-rinse, detergent wash, mechanical action, post-rinse, drying, and inspection. Specify what is included (all product contact parts, gaskets, hoses, utensils) and what is excluded. Define when disassembly is required and the extent of dismantling.
2) Detergent and solution parameters
Select the cleaning agent based on residue chemistry and material compatibility. Set detergent concentration range (e.g., % w/v), mixing method, solution volume, and maximum solution-use life. Define water quality for preparation (Purified Water/WFI if required) and temperature range, as both affect solubility and cleaning performance.
3) Mechanical action parameters (the “manual” criticals)
Standardize the scrubbing method: type of brush/pad (material, size), direction/pattern (crosshatch), number of strokes or minimum scrubbing time per surface area, and pressure guidance (where feasible). Identify “hard-to-clean” parts and require dedicated attention steps (valve seats, grooves, threads). Use visual aids/photos to reduce interpretation.
4) Contact time and dwell control
Set minimum detergent contact time and maximum time before rinsing to prevent drying or redeposition. Establish maximum “dirty hold time” before cleaning and “clean hold time” after cleaning, with storage conditions and protection (covered parts, clean area).
5) Rinsing parameters
Define rinse water quality, rinse temperature if relevant, and minimum rinse volumes or rinse cycles. Include criteria for final rinse (e.g., conductivity/TOC/pH within limits where applicable) to confirm detergent removal.
6) Drying and post-clean handling
Specify drying method (air dry, filtered compressed air), acceptable residual moisture, and reassembly requirements. Define clean labeling, storage, and prevention of recontamination.
7) Controls, documentation, and robustness
Create checklists, operator training/qualification, and second-person verification for critical steps. During validation, challenge the process at defined worst-case conditions (minimum detergent, minimum time/temperature) and confirm acceptance via visual inspection plus swab/rinse results against MACO/HBEL limits.





