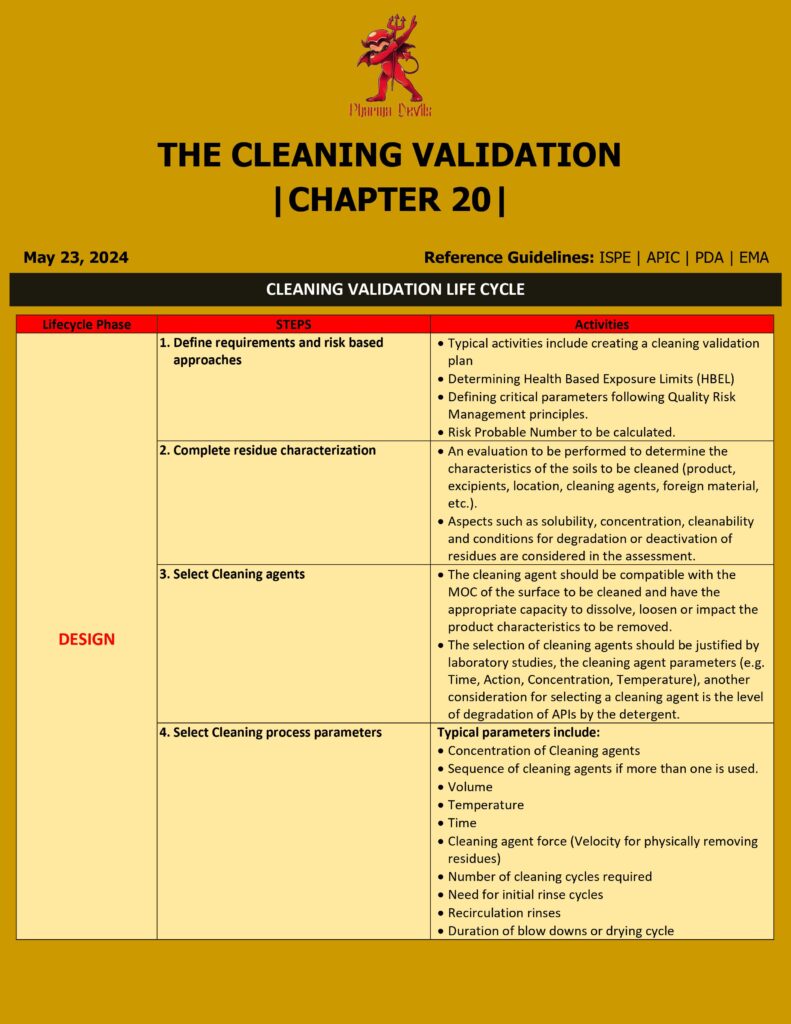



1. Process Design (Stage 1)
Goal: Design and understand the cleaning process so it can consistently remove product, detergent, and microbial residues to an acceptable level.
Key activities:
- Define scope
- Which equipment trains?
- Which products / worst-case products?
- Shared vs dedicated equipment?
- Risk assessment
- Identify worst-case products based on:
- Lowest therapeutic dose
- Highest toxicity
- Lowest solubility
- Sticky / highly adsorptive nature
- Identify hard-to-clean equipment parts (dead legs, gaskets, valves).
- Identify worst-case products based on:
- Set acceptance limits
- Health-based limits (HBEL / PDE-based MACO).
- Microbial & endotoxin limits (if applicable).
- Detergent residue limits.
- Choose and define cleaning process
- Manual vs automated (CIP/COP).
- Detergent type, concentration, contact time, temperature, number of rinse cycles.
- Equipment disassembly requirements.
- Analytical method development
- Swab and/or rinse sampling.
- Specific methods (HPLC/UPLC) vs non-specific (TOC, conductivity).
- Ensure methods are stability-indicating where needed.
2. Process Qualification / Validation (Stage 2)
Goal: Demonstrate and document that the designed cleaning process works as intended under routine conditions.
Typical steps:
- PQ protocol
- Define number of runs (often 3 consecutive successful runs per equipment train/worst-case).
- Define sampling locations (worst-case / hardest-to-clean locations).
- Define hold times (soils drying, dirty hold, clean hold).
- Execute cleaning validation runs
- Run the full cleaning procedure as per SOP.
- Document:
- Batch details (previous product, strength).
- Equipment IDs.
- Operators.
- Exact parameters (time, temp, detergent concentration).
- Sampling & testing
- Swab and/or rinse from defined locations.
- Visual inspection (no visible residues).
- Lab analysis vs acceptance criteria (API, degradants, detergent, bioburden, endotoxin).
- Evaluate data & conclude
- All results within limits.
- No trends toward failure.
- Document deviations and CAPA if any.
- Approve cleaning process and finalize validated status.
3. Continued / Ongoing Verification (Stage 3)
Goal: Maintain and demonstrate control over time, ensuring the cleaning process remains capable as products, equipment, or conditions change.
Ongoing activities:
- Routine monitoring
- Periodic verification (e.g., 1–2 runs per year per equipment train, or risk-based).
- Trending of results (e.g., TOC values, residual levels).
- Change control
- Re-assess Cleaning Validation when:
- New product (especially more toxic / lower dose).
- New equipment or surface material.
- Changes in cleaning agents, parameters, or SOP.
- Changes in batch size or campaign size.
- Re-assess Cleaning Validation when:
- Periodic review
- Review cleaning validation every 1–3 years:
- Trends, OOS / OOT.
- Deviations / incidents (cross-contamination, visible residues).
- Effectiveness of CAPAs.
- Confirm HBEL/PDE values are still appropriate with any new toxicology data.
- Review cleaning validation every 1–3 years:
- Continuous improvement
- Optimize:
- Reduced water / detergent usage.
- Shorter cleaning times.
- Better ergonomics / safety for operators.
- Implement new technology (e.g., better CIP design, new detergents, PAT/online monitoring).
- Optimize:
How it fits together (Simple View)
Life Cycle = Design → Qualify → Monitor & Improve
- Design the cleaning process
→ Understand risks, worst-cases, acceptance limits, and procedures. - Qualify / validate the process
→ Prove with data (multiple runs) that cleaning is effective and reproducible. - Verify & control over time
→ Periodic checks, change control, and continuous improvement.





