A compressed air system in pharma is a critical utility used for pneumatic valves/actuators, instrument air, cleanroom equipment, tablet presses, packaging machines, and blow-off/drying. Because air can contact product or product-contact surfaces indirectly, it must be designed and controlled to prevent contamination by oil, water, particles, microbes, and odors.
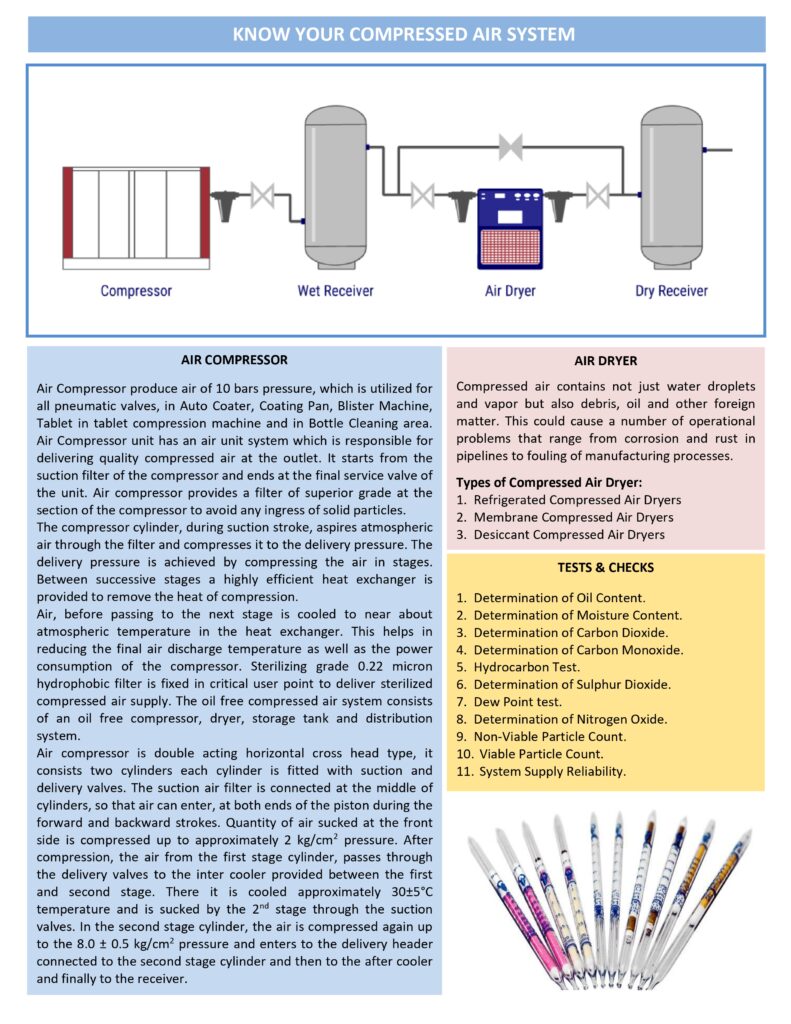
System overview (how it works)
Ambient air is drawn through an intake filter, compressed, cooled, dried, filtered, stored, and distributed to user points. The major design goal is delivering air at required pressure, flow, and quality consistently under worst-case demand.
Key components
- Air compressor (oil-free preferred for product contact / high purity needs; oil-lubricated can be used with robust downstream oil removal)
- Aftercooler + moisture separator to reduce water load
- Air receiver (buffer) with automatic drain
- Dryer: refrigerated or desiccant (desiccant commonly used for low dew points)
- Filtration train: particulate filter, coalescing filter (oil/water aerosols), activated carbon (vapors/odors), and sterile filter at point-of-use if required
- Distribution loop: sloped, drainable piping, minimal dead legs, drop legs with drains, and dedicated sampling points
Critical quality attributes
- Particles: controlled by rated filters (often to 0.01–1 µm depending on use)
- Oil: liquid + aerosol + vapor (must be minimized; carbon filter helps vapors)
- Moisture/Dew point: low dew point prevents condensation and microbial growth
- Microbial control: achieved by dryness, hygiene design, and sterile filtration for critical uses
- Pressure stability: avoids equipment malfunction and process variability
Qualification and monitoring
Perform DQ/IQ/OQ/PQ including piping slope/drainability checks, compressor performance, alarm/interlock verification, and air quality testing at worst-case points. Routine monitoring includes dew point, differential pressure across filters, compressor oil carryover (if applicable), particle/oil testing, and condensate drain function. Maintain calibration, preventive maintenance, and change control.
Common failures
Wet air from dryer issues, failed drains causing water carryover, saturated coalescing/carbon filters, leaks lowering pressure, microbial risks in stagnant lines, and poor point-of-use filtration selection. A controlled compressed air system protects product quality and GMP compliance.





