


PERSONNEL ASEPTIC QUALIFICATION COMPILED REPORT
BY RODAC (CONTACT PLATE) METHOD
1.0 REPORT DETAILS
- Report Title: Personnel Aseptic Qualification by RODAC Plate Method
- Report No.: PQ/RODAC/2025/017
- Related Protocol No.: PQ/RODAC/2025/006
- Department: Sterile Manufacturing – Production
- Area: Aseptic Filling – Grade B/A
- Report Date: 20-Nov-2025
2.0 OBJECTIVE
The objective of this report is to summarize the execution and results of aseptic personnel qualification in pharma performed using the RODAC (contact) plate method, and to conclude whether the candidate meets the predefined acceptance criteria for working in the aseptic area (Grade B/A).
3.0 SCOPE
This report covers the aseptic qualification, by RODAC plate method, of the following personnel as per Protocol No. PQ/RODAC/2025/006:
- Name: Mr. Arjun Sharma
- Employee ID: EMP-0412
- Designation: Production Officer – Aseptic Filling
Area of qualification: Sterile Injectables Aseptic Filling – Grade B/A.
4.0 REFERENCES
- Protocol: Personnel Gowning & Aseptic Behaviour Qualification Using RODAC Plate Method
Protocol No.: PQ/RODAC/2025/006 - SOP for Gowning into Aseptic Area: SOP No. SMF/SOP/GWN/004
- SOP for Environmental Monitoring by Contact Plates: SOP No. SMF/SOP/EM/006
- Environmental Monitoring Program & Limits: Document No. SMF/QC/EM/PLAN/2025/01
5.0 SUMMARY OF EXECUTION
- Protocol Issue Date: 30-Oct-2025
- Execution Period (for this candidate): 05-Nov-2025 to 09-Nov-2025
- Trials Planned per Candidate: 3 consecutive trials
- Trials Executed: 3 trials
Execution summary:
Personnel aseptic qualification for Mr. Arjun Sharma (EMP-0412) was carried out as per the approved protocol PQ/RODAC/2025/006. The candidate performed three consecutive gowning trials on separate days under supervision. RODAC contact plate sampling was performed from predefined gown and glove locations after gowning. Plates were incubated and read as per the Environmental Monitoring Program. All results were compared against the predefined acceptance criteria.
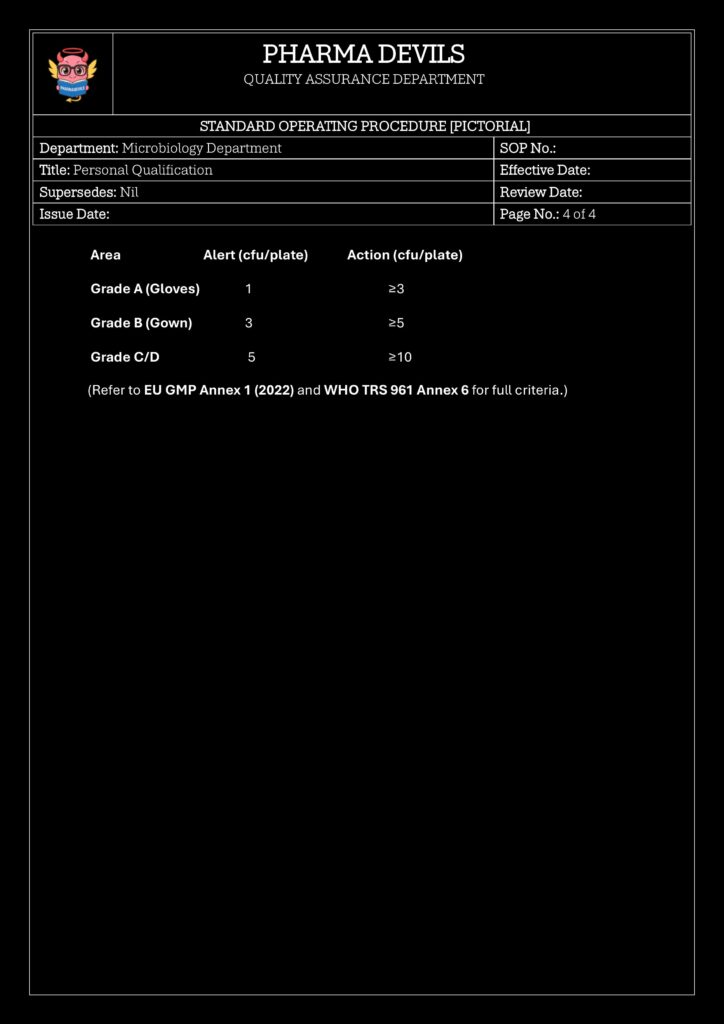
6.0 ACCEPTANCE CRITERIA (SUMMARY)
- Three (3) consecutive trials must comply with gowning microbiological limits.
- Gloved hands (each):
- Action / Failure limit: > 0 CFU/plate (any growth = trial failure).
- Other gown sites (sleeves, chest, back, knee):
- Action / Failure limit: ≥ 3 CFU/plate.
- No growth of objectionable organisms (e.g. Gram-negative rods, heavy mold).
Only candidates who meet these criteria in three consecutive trials are considered Qualified for entry and work in the aseptic area (Grade B/A).
7.0 INDIVIDUAL PERSONNEL RESULTS
7.1 Candidate Details
- Name: Mr. Arjun Sharma
- Employee ID: EMP-0412
- Designation/Role: Production Officer – Aseptic Filling
- Department: Sterile Manufacturing – Production
7.2 Trial Dates
| Trial No. | Date | Observer (Microbiology/QA) | Remarks |
|---|---|---|---|
| Trial 1 | 05-Nov-2025 | Ms. Pooja Nair (QC Micro) | No visible gowning errors. |
| Trial 2 | 07-Nov-2025 | Mr. R. Tiwari (QA) | Gowning performed smoothly. |
| Trial 3 | 09-Nov-2025 | Ms. Pooja Nair (QC Micro) | No intervention required. |
7.3 RODAC Results per Trial
Sampling sites (per trial):
- Gloved Right Hand – Fingers & Palm
- Gloved Left Hand – Fingers & Palm
- Right Forearm Sleeve
- Left Forearm Sleeve
- Chest (Front of Gown)
- Back/Shoulder Area
- Knee / Leg Area
Acceptance reference:
- Gloves: 0 CFU/plate required
- Gown sites: Action limit ≥ 3 CFU/plate
7.3.1 Trial 1 – RODAC Results (05-Nov-2025)
| Sampling Site | Action Limit (CFU/plate) | Result (CFU/plate) | Pass/Fail | Remarks |
|---|---|---|---|---|
| Gloved Right Hand | >0 = Fail | 0 | Pass | – |
| Gloved Left Hand | >0 = Fail | 0 | Pass | – |
| Right Forearm Sleeve | ≥3 = Fail | 0 | Pass | – |
| Left Forearm Sleeve | ≥3 = Fail | 1 | Pass | Within alert; acceptable. |
| Chest | ≥3 = Fail | 0 | Pass | – |
| Back/Shoulder | ≥3 = Fail | 0 | Pass | – |
| Knee / Leg Area | ≥3 = Fail | 0 | Pass | – |
Overall Trial 1 Result: PASS
No unusual colony morphologies were observed. No objectionable organisms detected.
7.3.2 Trial 2 – RODAC Results (07-Nov-2025)
| Sampling Site | Action Limit (CFU/plate) | Result (CFU/plate) | Pass/Fail | Remarks |
|---|---|---|---|---|
| Gloved Right Hand | >0 = Fail | 0 | Pass | – |
| Gloved Left Hand | >0 = Fail | 0 | Pass | – |
| Right Forearm Sleeve | ≥3 = Fail | 0 | Pass | – |
| Left Forearm Sleeve | ≥3 = Fail | 0 | Pass | – |
| Chest | ≥3 = Fail | 1 | Pass | Within alert; acceptable. |
| Back/Shoulder | ≥3 = Fail | 0 | Pass | – |
| Knee / Leg Area | ≥3 = Fail | 0 | Pass | – |
Overall Trial 2 Result: PASS
No unusual or objectionable organisms observed.
7.3.3 Trial 3 – RODAC Results (09-Nov-2025)
| Sampling Site | Action Limit (CFU/plate) | Result (CFU/plate) | Pass/Fail | Remarks |
|---|---|---|---|---|
| Gloved Right Hand | >0 = Fail | 0 | Pass | – |
| Gloved Left Hand | >0 = Fail | 0 | Pass | – |
| Right Forearm Sleeve | ≥3 = Fail | 0 | Pass | – |
| Left Forearm Sleeve | ≥3 = Fail | 0 | Pass | – |
| Chest | ≥3 = Fail | 0 | Pass | – |
| Back/Shoulder | ≥3 = Fail | 0 | Pass | – |
| Knee / Leg Area | ≥3 = Fail | 1 | Pass | Within alert; acceptable. |
Overall Trial 3 Result: PASS
No unusual colony morphologies were seen. No objectionable organisms detected.
7.4 Deviations / OOS / Atypical Findings
- Deviation / OOS ID: Not applicable
- Description: No deviations or OOS results were reported during sampling, incubation or reading of plates.
- Root Cause: Not applicable
- CAPA: Not applicable
- Impact on Qualification: None
7.5 Final Qualification Status – Candidate
- Candidate: Mr. Arjun Sharma (EMP-0412)
Summary:
- Completed three (3) consecutive trials on separate days.
- All glove samples showed 0 CFU/plate.
- All gown sites were below action limits, with occasional values of 1 CFU/plate within alert range.
- No objectionable organisms were isolated.
Final Status: QUALIFIED for entry and work in Aseptic Area (Grade B/A) – Sterile Injectables Filling.
Evaluator (QC Microbiology):
Name: Ms. Pooja Nair
Designation: Executive – QC Microbiology
Signature: _____________________
Date: 18-Nov-2025
Reviewer (Quality Assurance):
Name: Mr. Ashok Mehta
Designation: Executive – QA
Signature: _____________________
Date: 19-Nov-2025
8.0 OVERALL EVALUATION & DISCUSSION
During this qualification exercise, Mr. Arjun Sharma demonstrated consistent adherence to aseptic gowning and behaviour requirements. All three trials were completed without procedural deviations. Microbiological results from RODAC plates remained well within the predefined acceptance criteria, with no growth on glove samples and only occasional 1 CFU readings on non-critical gown locations (sleeves, chest or knee), which remained within alert limits.
No objectionable organisms were detected across any of the trials. The results suggest good control of personal hygiene, hand washing, and gowning technique. No additional CAPA was required beyond routine training reinforcement.
The overall program for this candidate is considered effective.
9.0 CONCLUSION
Based on:
- Successful completion of three consecutive aseptic gowning trials,
- Compliance with RODAC plate acceptance criteria for all sampling sites, and
- Absence of objectionable organisms and deviations,
Mr. Arjun Sharma (EMP-0412) is hereby qualified to work in the Aseptic Area (Grade B/A) of Sterile Injectables Manufacturing as a Production Officer – Aseptic Filling.
This qualification remains valid until the next scheduled requalification, or earlier if triggered by relevant deviations, EM trends, or extended absence from aseptic activities.
10.0 APPROVALS
Prepared By (QC Microbiology / Validation):
Name: Ms. Pooja Nair
Designation: Executive – QC Microbiology
Signature: __________________________
Date: 18-Nov-2025
Reviewed By (Production / User Department):
Name: Mr. R. Tiwari
Designation: Manager – Production (Sterile)
Signature: __________________________
Date: 19-Nov-2025
Reviewed By (Quality Assurance):
Name: Mr. S. Mehta
Designation: Executive – QA
Signature: __________________________
Date: 19-Nov-2025
Approved By (Head – Quality Assurance):
Name: Mr. B. Mehra
Designation: Head – Quality Assurance
Signature: __________________________
Date: 20-Nov-2025





