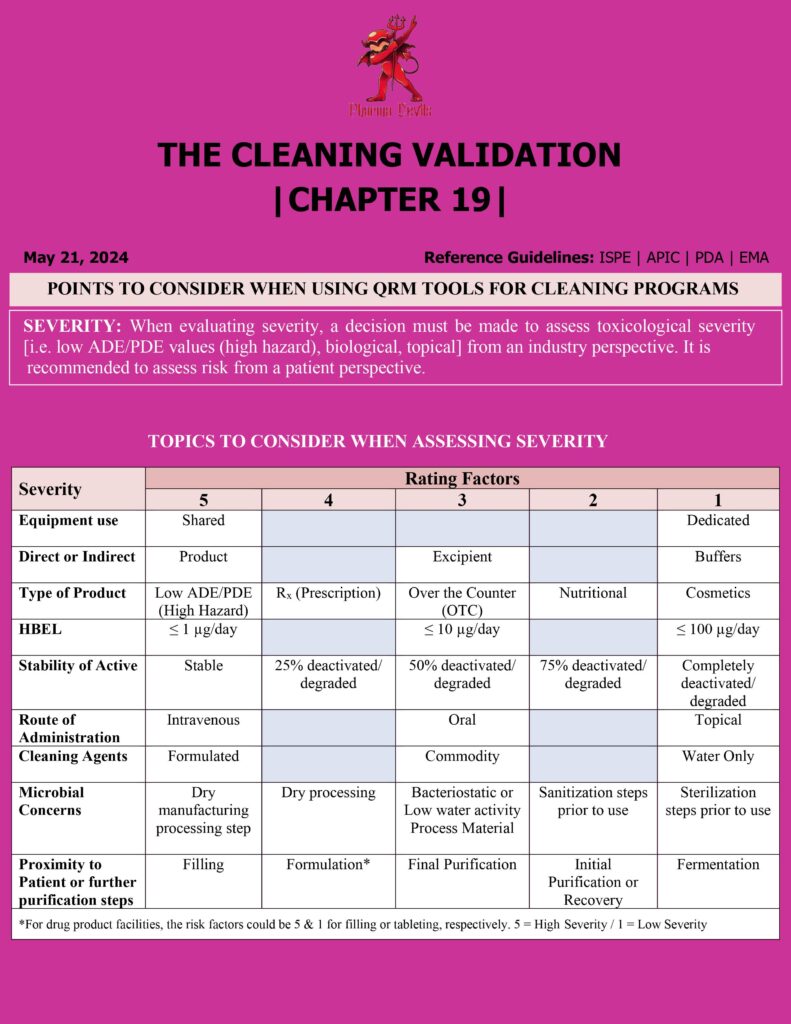
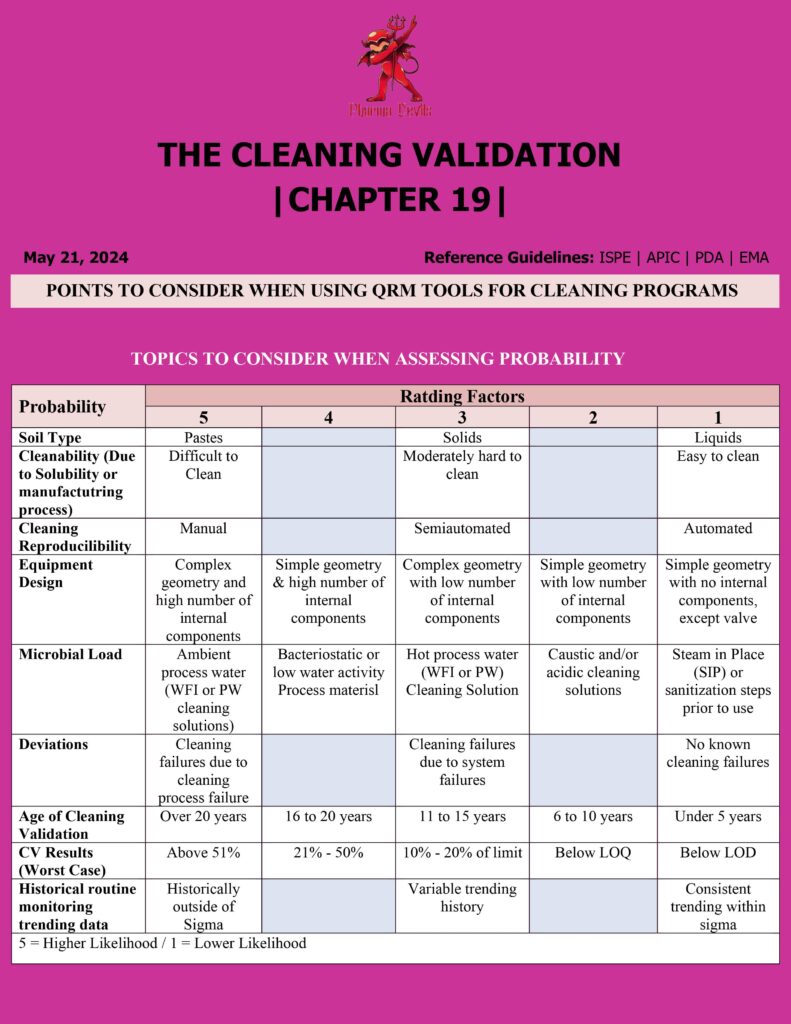
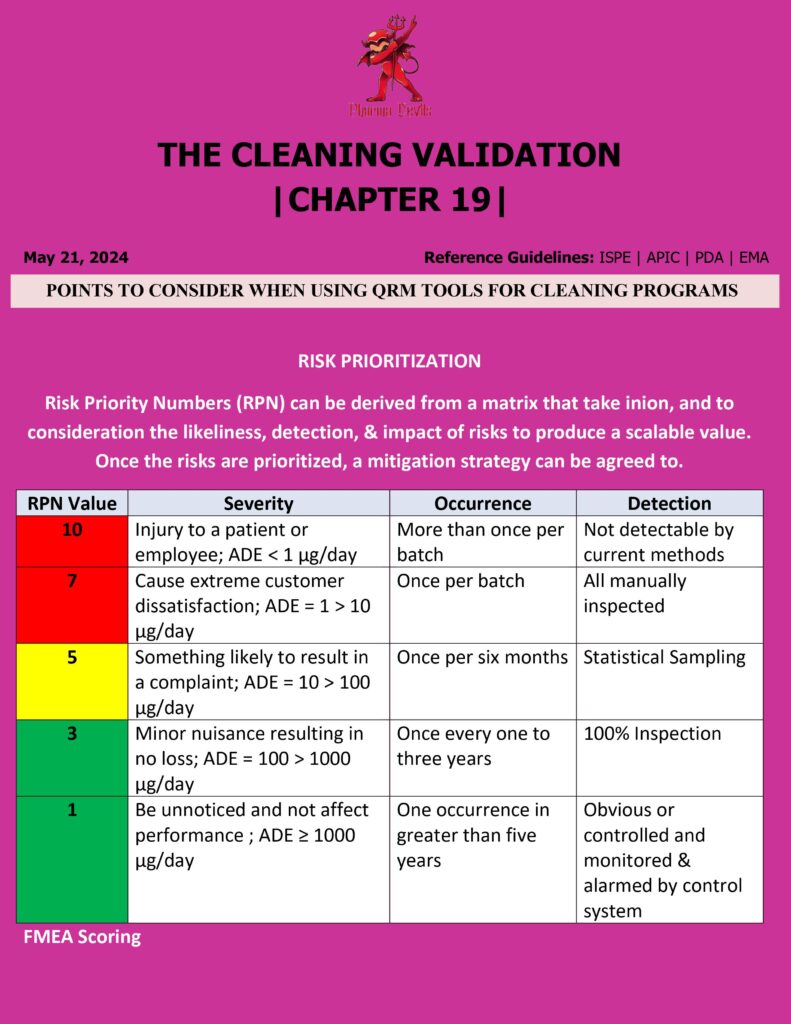
Key Points for Applying QRM Tools in Pharma Cleaning Programs
Applying Quality Risk Management (QRM) tools to cleaning programs helps pharma companies design and maintain risk-based, scientifically sound and compliant cleaning strategies. Instead of treating all products, equipment and residues equally, QRM focuses attention on what truly matters for patient safety and product quality.
1. Understand the Cleaning Risks
Start by clearly defining what can go wrong if cleaning is ineffective:
- Cross-contamination between products or strengths
- Microbial contamination or biofilm development
- Degradation residues or cleaning agent carryover
List all potential hazards associated with residues (toxicity, potency, solubility, dose, therapeutic index) and with the equipment design (dead legs, complex parts, internal surfaces, filters, transfer lines).
2. Select Appropriate QRM Tools
Choose QRM tools that match the complexity and impact of the cleaning process, such as:
- Checklists and flowcharts for simple qualitative assessments
- Risk ranking and filtering for prioritizing products and equipment
- FMEA (Failure Mode and Effects Analysis) or similar structured tools for high-risk systems
The goal is not to overload documentation, but to generate clear, justified decisions.
3. Define Risk Criteria and Acceptance Levels
Before using QRM tools, define:
- Severity, occurrence and detectability scales
- Acceptance criteria for risk scores
- Links between risk level and control/validation strategy
This ensures consistency, avoids subjective scoring and supports regulatory review.
4. Link Risks to Cleaning Validation and Controls
Use QRM outcomes to decide:
- Which products/equipment require full cleaning validation or grouping/bracketing
- Sampling locations and methods (swab, rinse, visual)
- Limits for residues based on health-based exposure limits (HBELs) or PDE values
- Frequency of revalidation and monitoring
5. Document, Review and Update
All QRM activities must be well documented, justified and approved by cross-functional teams (QA, Production, QC, Engineering). Regularly review risk assessments when there are new products, process changes, deviations or inspection comments.
By systematically applying QRM tools, cleaning programs become more focused, defendable and effective, supporting both regulatory compliance and robust patient protection.





