Risk Based Inspection (RBI) – Schedule M Point 4.13 checks “What is the process for preparation of Water for Injection (WFI)?” and expects a clear, validated, GMP-controlled description from PW feed to WFI storage/distribution. CDSCO
Under Schedule M (sterile products – Water & Steam Systems), WFI must be prepared from potable water or purified water meeting required specifications by distillation, and the WFI must meet microbiological and endotoxin limits and applicable pharmacopoeial requirements. Because WFI is used for high-risk operations (bulk preparation, final rinses, disinfectant preparation), RBI treats 4.13 as a critical utility: any weakness can lead to microbial/endotoxin contamination, batch impact, and patient harm.
What inspectors look for (practical RBI expectations)
- End-to-end process description
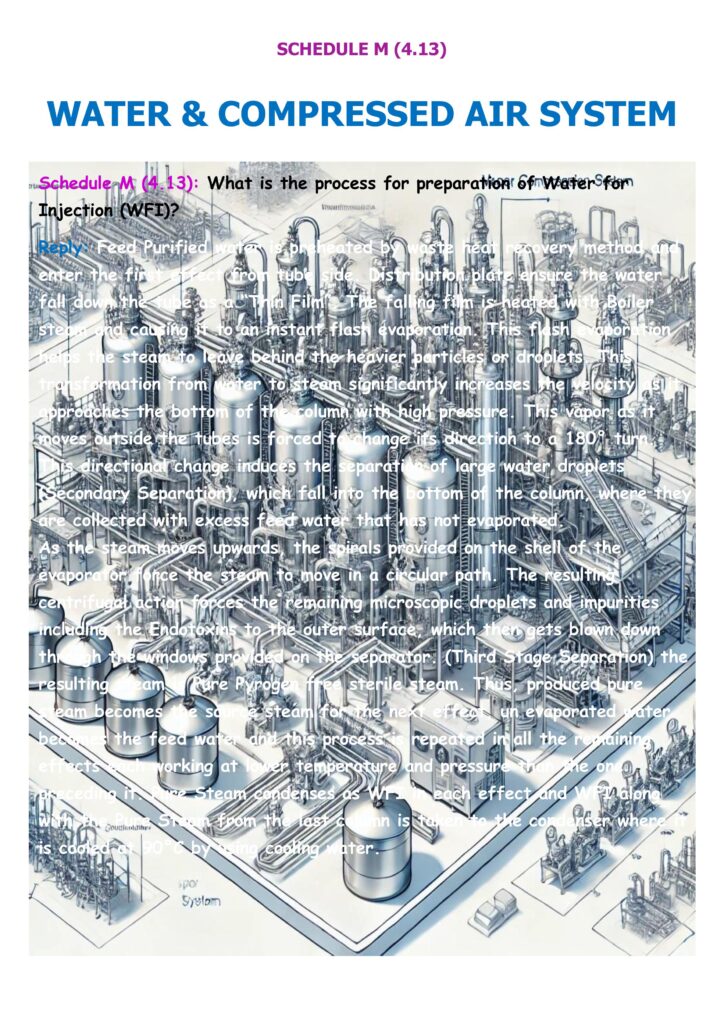
Inspectors expect the firm to describe the WFI generation train: raw water → pretreatment (softener/RO/EDI as applicable) → Purified Water → distillation system (commonly multi-column/multi-effect) → WFI storage → circulation loop and points of use. - Typical compliant example (as shown in RBI scoring guidance)
A commonly accepted description is: Purified water is fed into a multicolumn distillation plant, often fitted with online TOC and conductivity monitoring and PLC control; the WFI is then stored in a steam-jacketed SS316/SS316L vessel with constant circulation at elevated temperature to control bioburden. - Validation/qualification evidence
RBI verifies DQ/IQ/OQ/PQ (or equivalent qualification package) for the distiller and loop, including: performance at worst-case demand, controls/alarms, instrument calibration, and preventive maintenance. The inspector also checks whether deviations (e.g., conductivity/TOC/micro excursions) trigger investigation, batch impact assessment, and CAPA. - Control strategy & monitoring
The process must define: feed-water acceptance, operating parameters (temperatures, flows, pressures), sampling points, routine testing (conductivity, TOC, bioburden, endotoxin), trending with alert/action limits, and data integrity for electronic records.
A strong 4.13 response is concise but complete: it demonstrates distillation-based WFI generation, continuous control, validated performance, and robust monitoring from source to point-of-use.





