Risk-Based Inspection (RBI) is a GMP approach where inspectors decide the frequency, depth and breadth of inspection using Quality Risk Management, focusing more effort on systems that can most impact patient safety and product quality. CDSCO
In the RBI checklist, Schedule M points 8.13–8.14 (and the linked WHO TRS 986 point 8.15) target primary packaging material storage and sampling controls—because failures here can cause mix-ups, contamination, and defective container-closure performance (e.g., leakers, particulate, extractables/compatibility issues), directly affecting product quality.
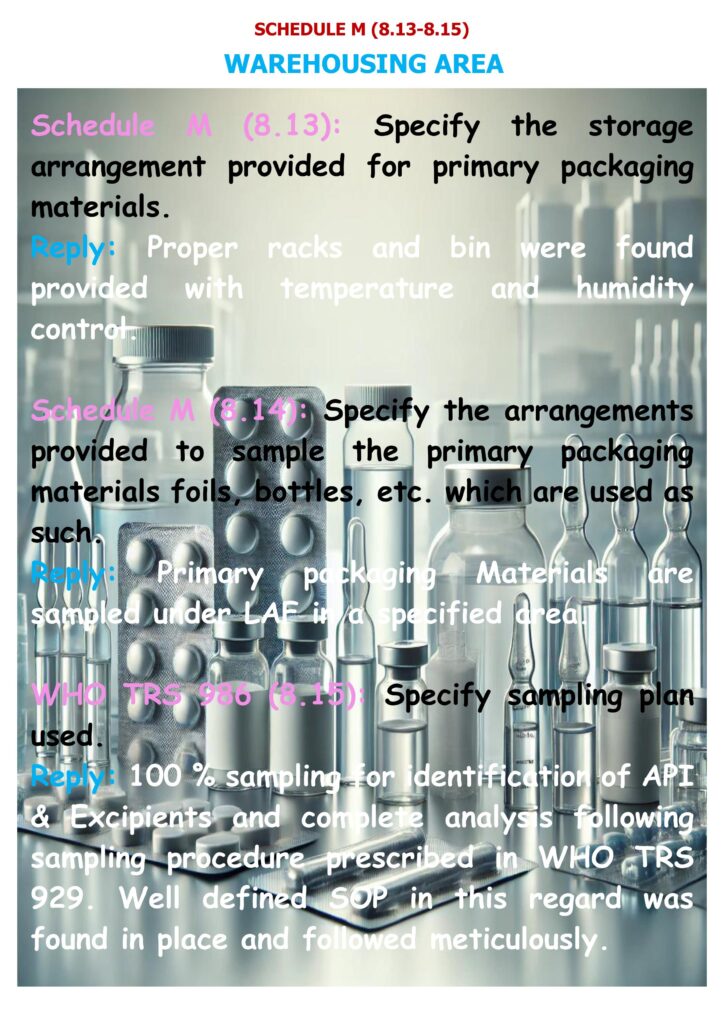
8.13: Storage arrangement for primary packaging materials
The requirement is to specify the storage arrangement provided for primary packaging materials (e.g., bottles, vials, stoppers, caps, foils, blister base, ampoules).
During RBI, inspectors typically verify:
- Segregation & identification: dedicated zones/racks/bins; clear labels with material name/code, supplier lot, internal status (quarantine/released/rejected), and traceability to purchase/CoA.
- Environmental protection: control of temperature/humidity/dust as needed for the material; protection from sunlight and physical damage (crushing/warping of foils, deformation of plastics).
- Cleanliness & contamination prevention: closed shippers, pallet hygiene, no mixed storage with raw materials that can shed dust/odour; pest control and housekeeping effectiveness.
- Security & mix-up prevention: restricted access for printed primary components (if applicable) and robust FEFO/FIFO to avoid outdated components.
Any observation like “primary packing stored with secondary packs or raw materials” is treated as a risk signal because it increases mix-up and contamination probability.
8.14–8.15: Sampling arrangements and sampling plan
Schedule M requires you to specify arrangements provided to sample primary packaging materials (foils, bottles, etc.) used as such. The checklist then asks to specify the sampling plan used (WHO TRS 986).
RBI checks that sampling is:
- Performed in a defined sampling area (often with controlled air and LAF where justified), using clean tools, and preventing introduction of fibers/dust.
- Governed by a written sampling plan (risk/statistics-based), defining sample quantity, acceptance criteria, special controls for critical defects (cracks, pinholes, particulate, dimensional checks), and actions for failures (quarantine, investigation, supplier feedback, CAPA).
Strong compliance shows storage + sampling controls that are designed, documented, implemented, and trended—not just “materials kept in store.”





