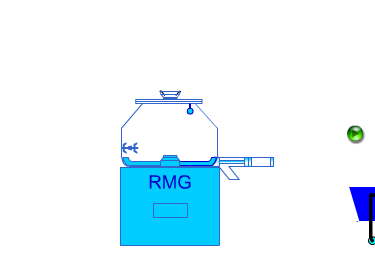
LAYOUTS IN PHARMA

In the pharmaceutical industry, layouts play a critical role in ensuring compliance with GMP, product quality, operational efficiency, and contamination control. Different areas of a pharmaceutical facility require different types of layouts depending on the type of manufacturing (sterile vs.…