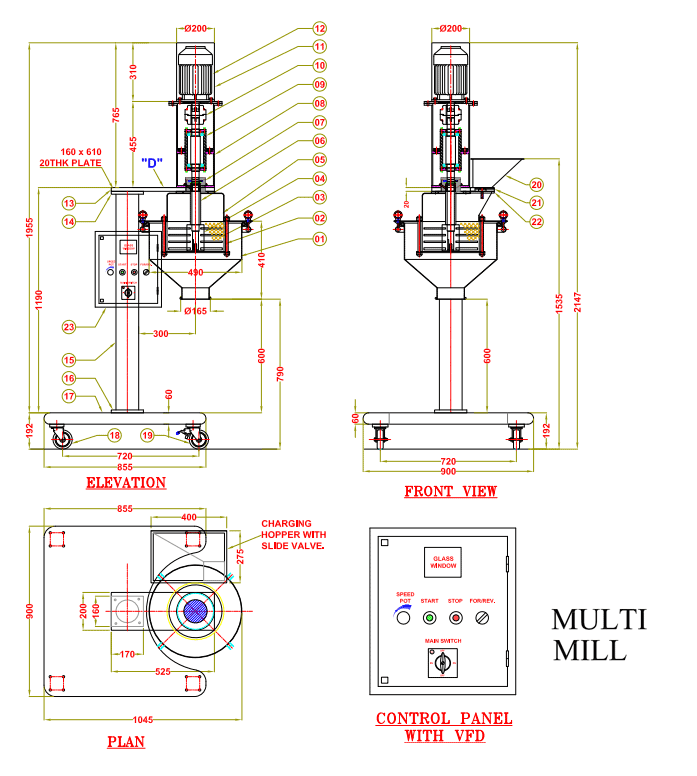
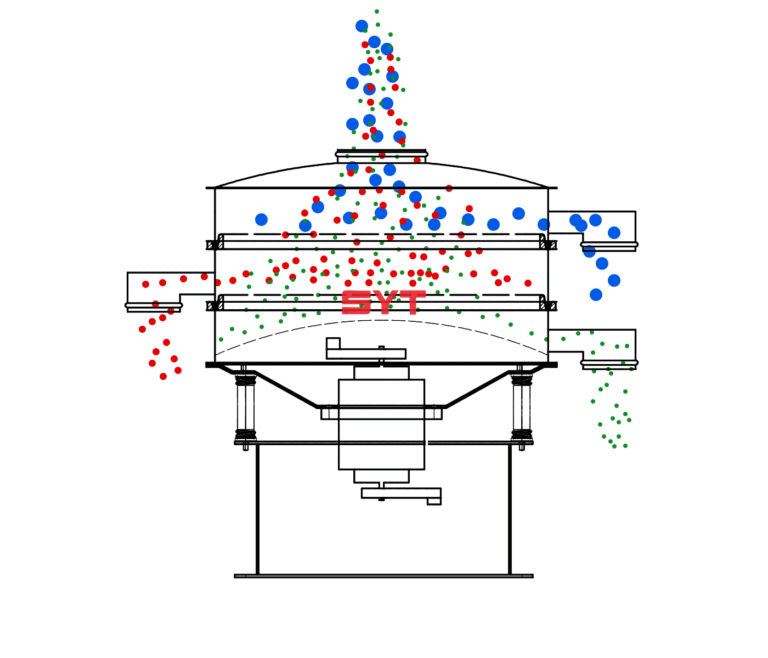
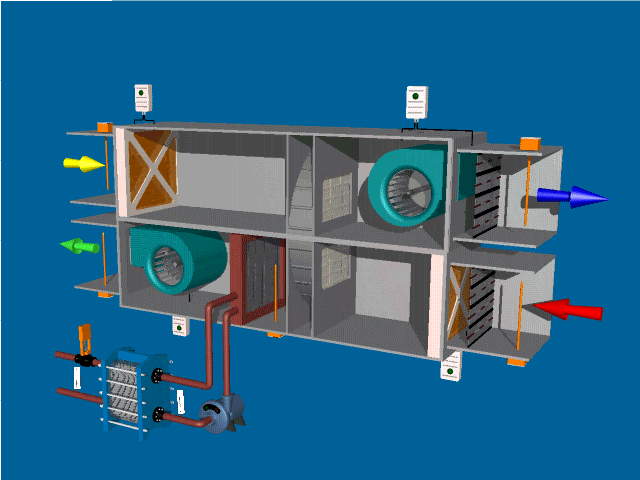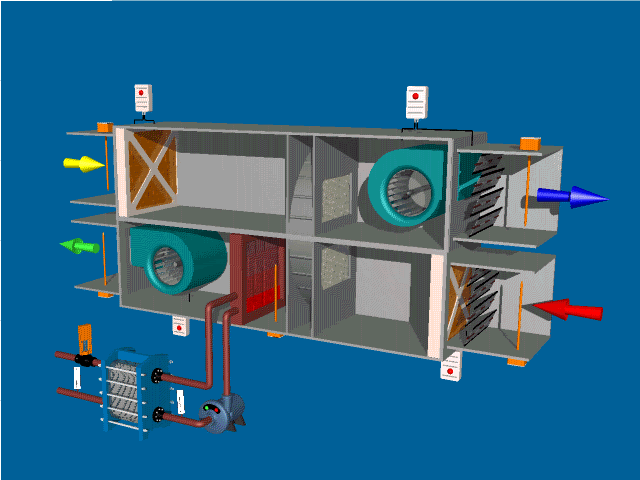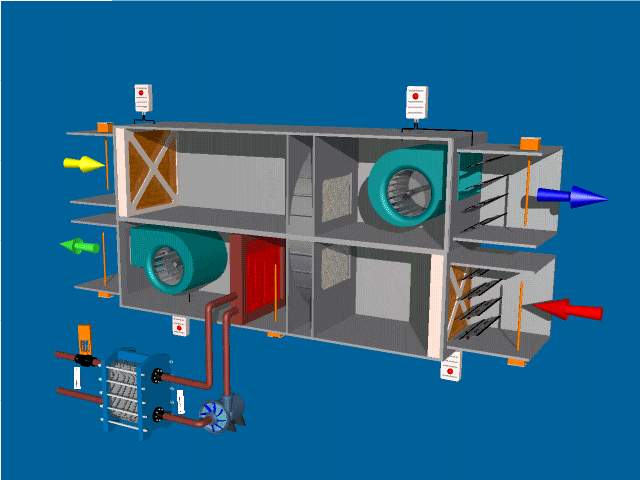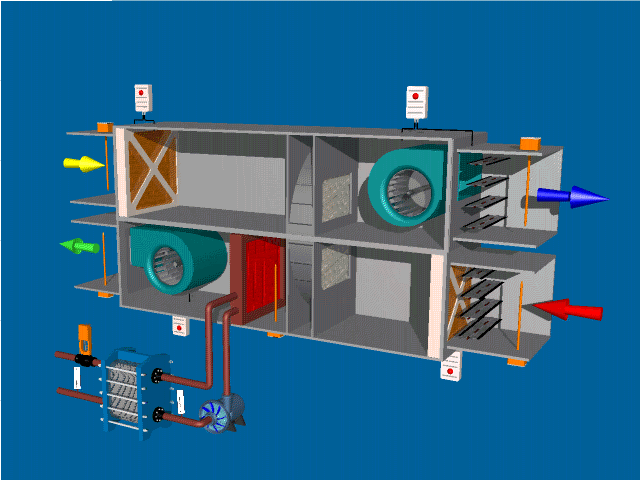
SELF-INSPECTION IN PHARMA

Self-inspection in the pharmaceutical industry is a critical aspect of Good Manufacturing Practices (GMP) compliance. It involves an internal audit conducted by a pharmaceutical company to evaluate its processes, facilities, and systems to ensure they meet regulatory requirements and internal…