DECODING WAREHOUSE (CHAPTER 1: DE-DUSTING TUNNEL)
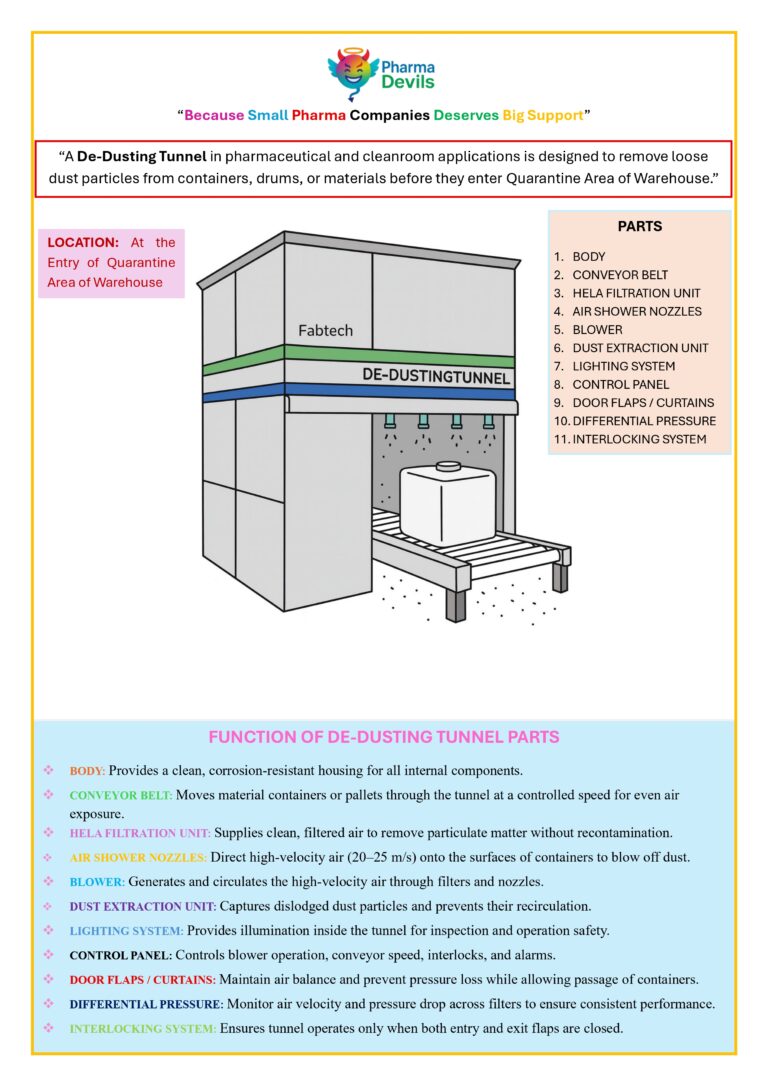
De-Dusting Tunnel in Pharmaceutical Warehouse A de-dusting tunnel in a pharmaceutical warehouse is a controlled system designed to remove loose dust, dirt, and external contamination from shipper cartons, drums, and other outer containers before they enter classified or controlled areas.…














