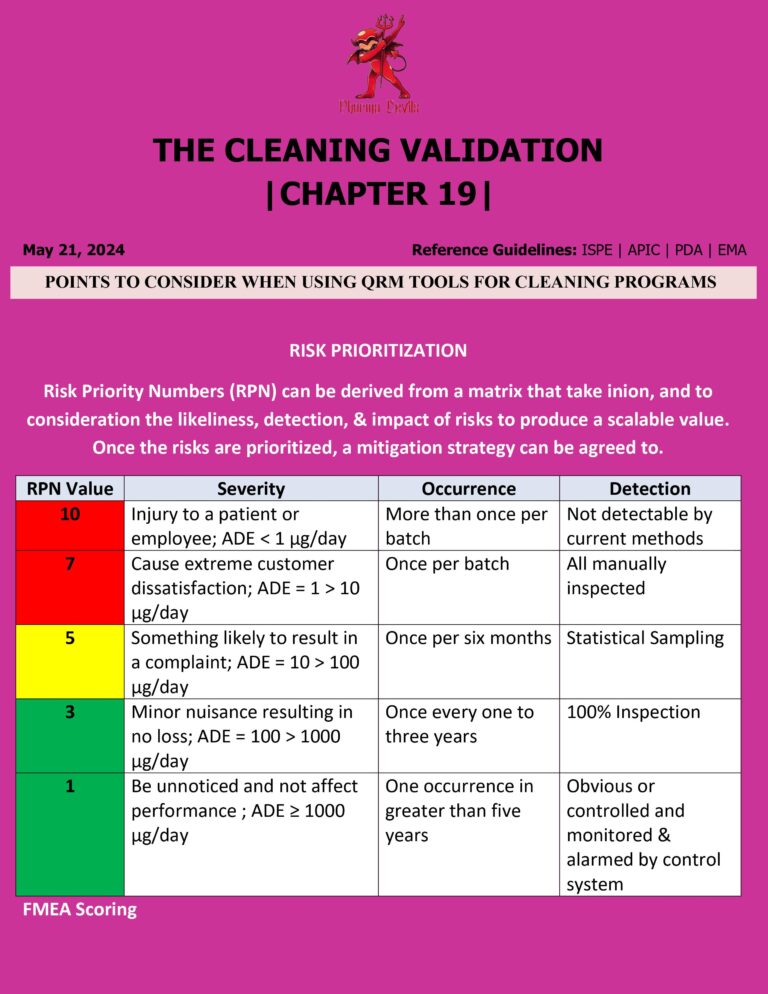
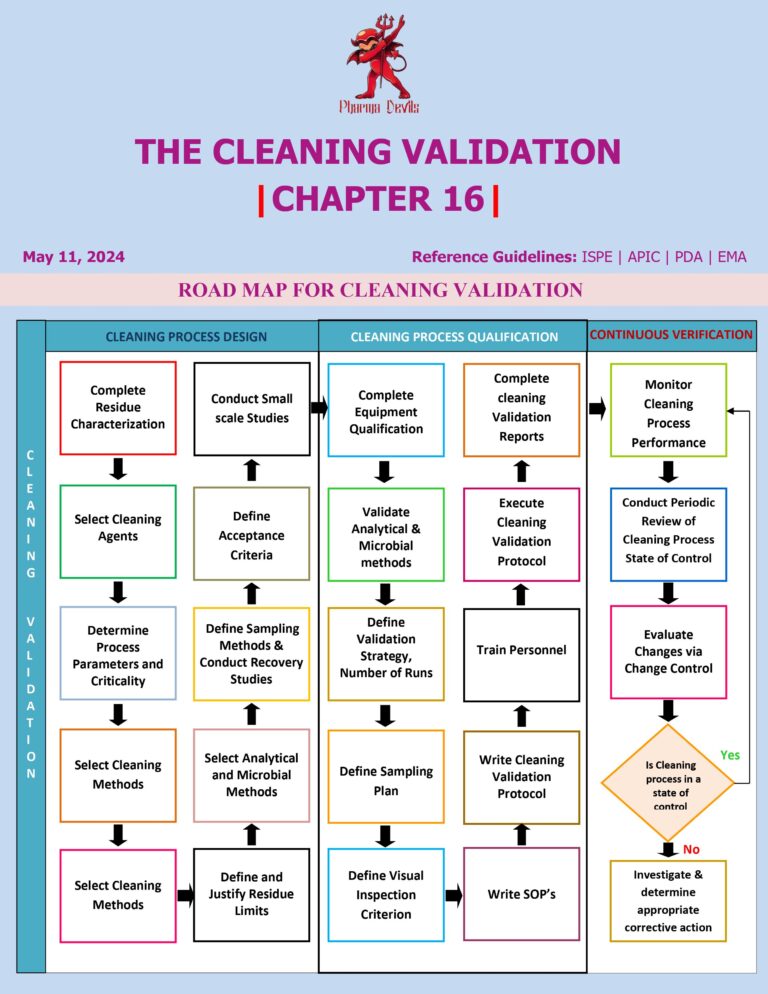
Cleaning Validation Life Cycle in Pharma
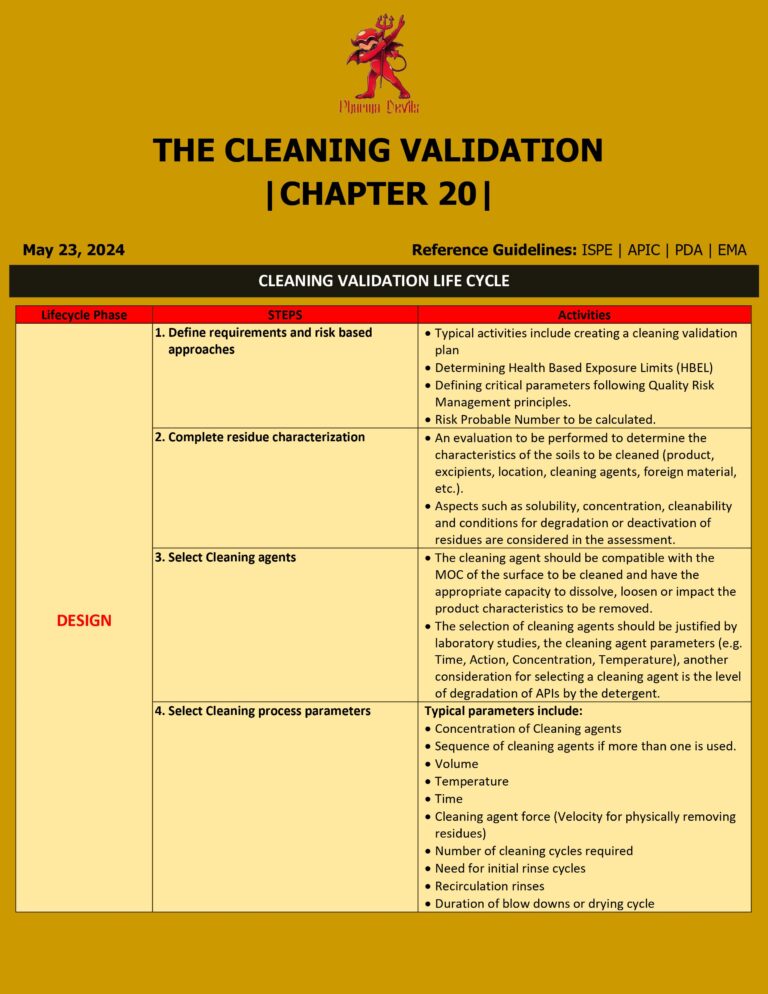
1. Process Design (Stage 1) Goal: Design and understand the cleaning process so it can consistently remove product, detergent, and microbial residues to an acceptable level. Key activities: 2. Process Qualification / Validation (Stage 2) Goal: Demonstrate and document that…