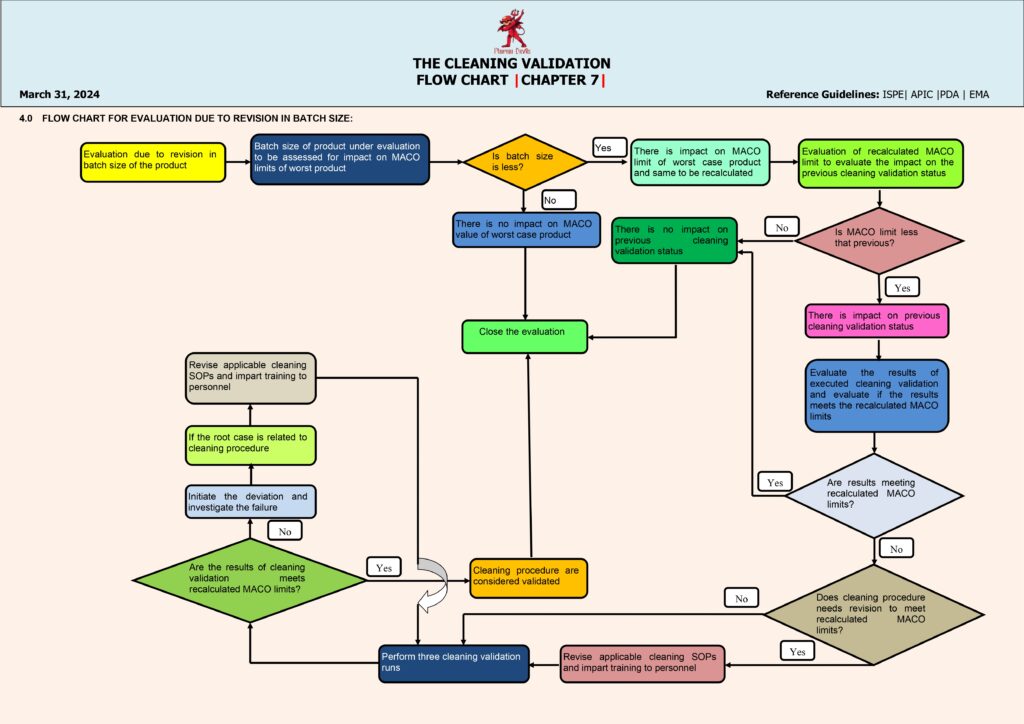
Cleaning Validation Flow (Pharmaceutical Manufacturing)
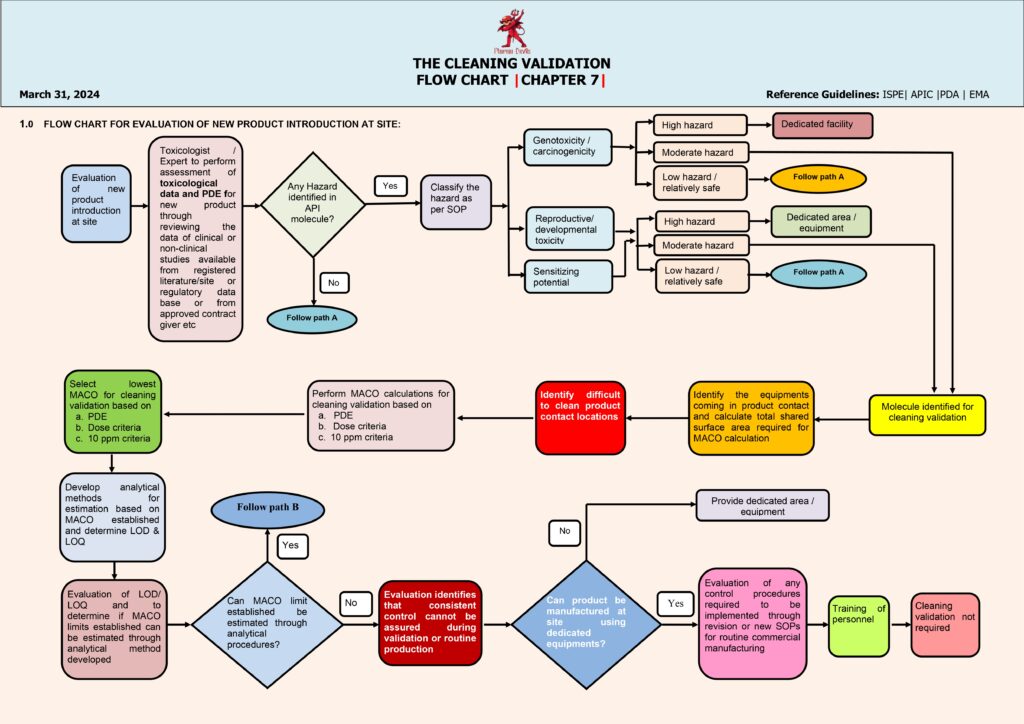
Cleaning validation is a documented program that demonstrates that equipment cleaning procedures consistently remove product residues, cleaning agents, and microbial contaminants to pre-defined acceptable levels. The flow begins with scope and equipment selection, typically focusing on shared, multi-product equipment and worst-case risk areas (hard-to-clean parts, long hold times, high potency/toxicity products). Next is process understanding and risk assessment, where worst-case product is chosen based on factors like lowest therapeutic dose, least soluble, most sticky, highest toxicity, and smallest batch size.
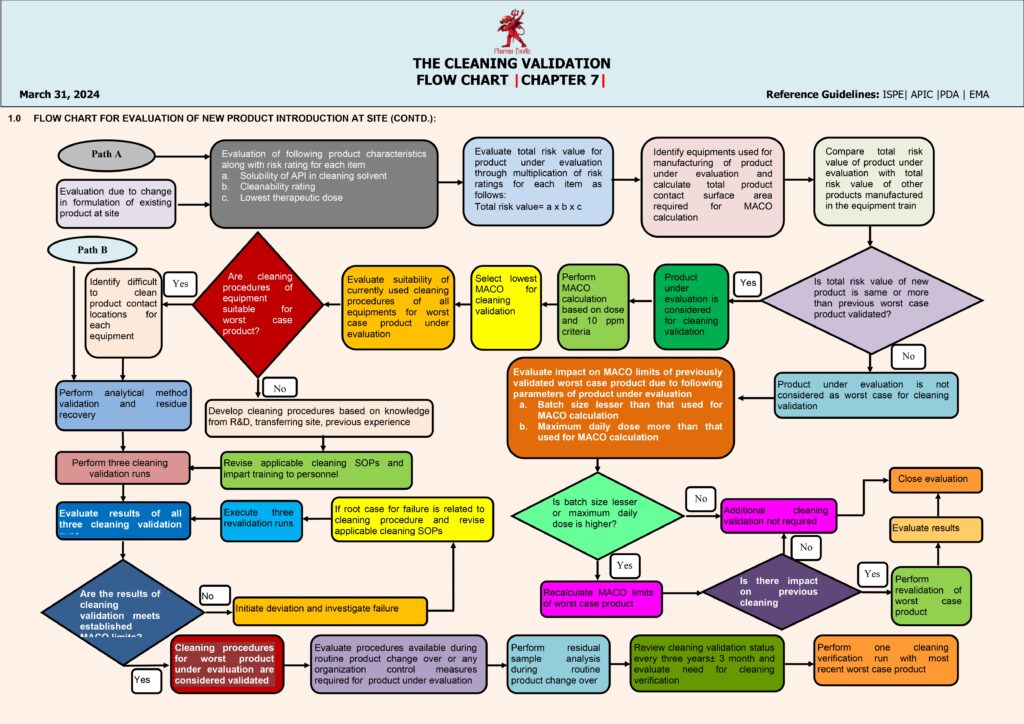
The next step is defining the cleaning procedure (CIP/COP/manual), including detergent type, concentration, contact time, temperature, rinse quality, utilities, and disassembly instructions. Then teams establish acceptance criteria, usually using a combination of: (1) MACO (Maximum Allowable Carryover) calculated from dose/toxicology limits, (2) visual cleanliness, and (3) microbial/endotoxin limits where applicable. A sampling plan is created: swab locations represent worst-case contact points (crevices, gaskets, dead legs) and rinse sampling is used where swabbing is not feasible. Analytical methods are selected and validated (e.g., HPLC/TOC/conductivity, specific detergent tests), ensuring suitable recovery studies for swabs.
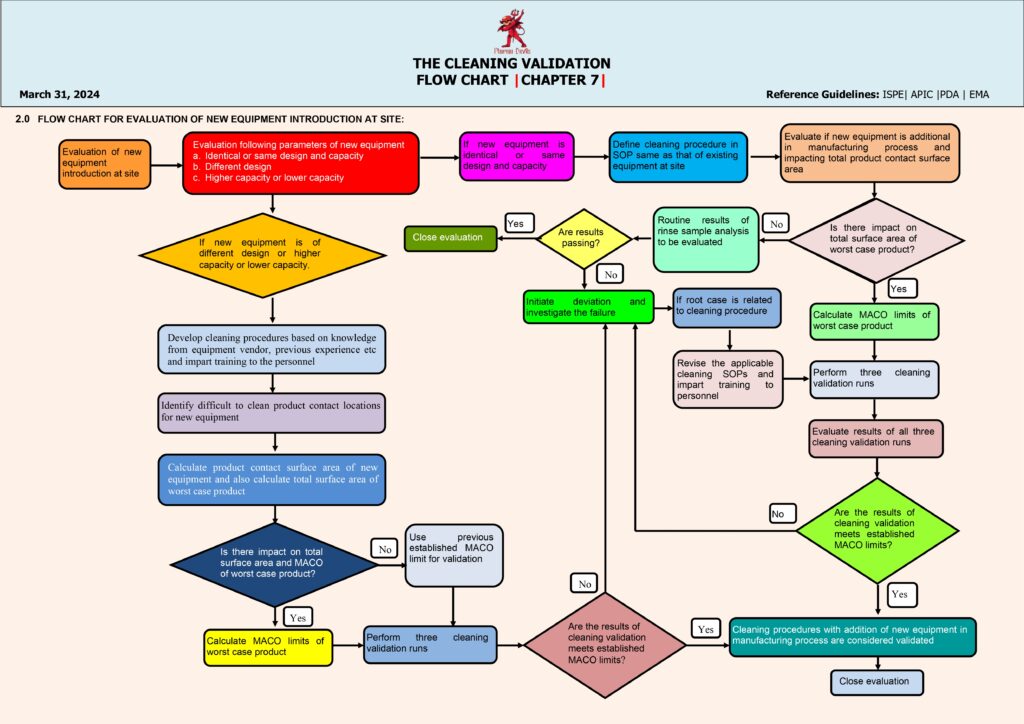
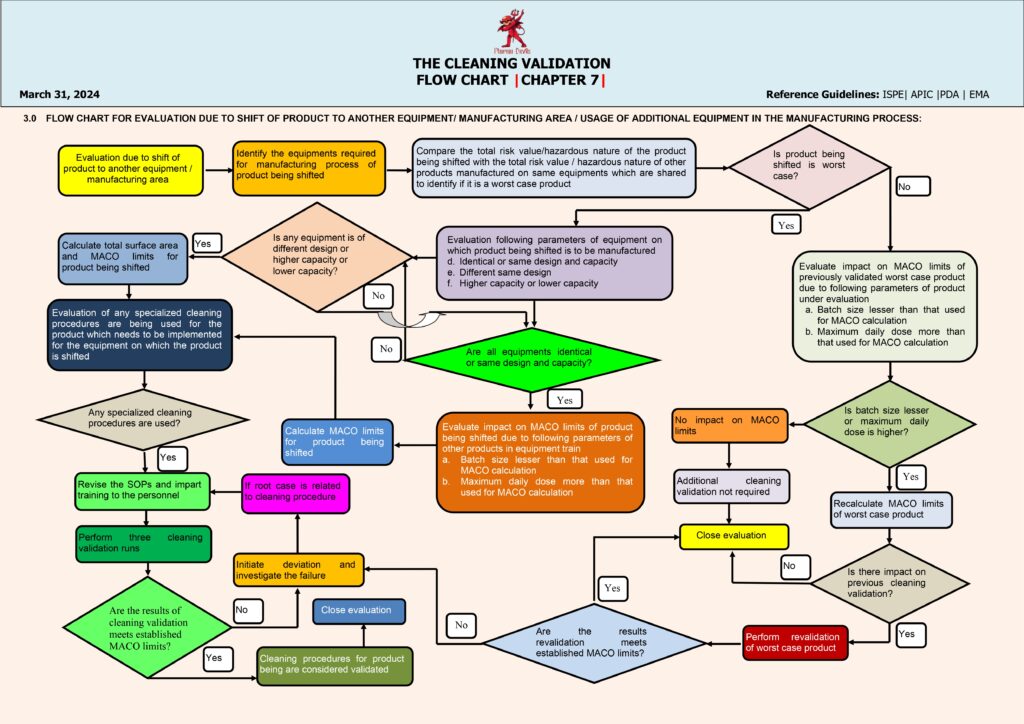
Execution moves to qualification runs—typically three consecutive successful cleaning runs under routine conditions—covering product changeovers and relevant dirty/clean hold times. Samples are tested, results are reviewed, and any failure triggers a deviation investigation, root cause analysis, corrective actions, and re-validation if needed. When all criteria are met, a Cleaning Validation Report is approved, and the process transitions to ongoing verification, including periodic monitoring, change control (new product, detergent, equipment modification), and revalidation triggers. This flow ensures patient safety, prevents cross-contamination, and maintains GMP compliance.






I.kamarajan
Sr.GM QA/QC
Vartika chemicals and pharmaceuticals pvt ltd
Bhiwadi, Rajasthan
HI
Very Good Flow Chart. Easy and self explainatory.
Thanks