

Daily balance verification in pharma is the routine, documented checking of analytical balances each day they’re used to ensure they are accurate and fit for use in GMP testing. It’s a pre-use fitness check, not a full calibration.
Here’s a crisp, SOP-style explanation you can directly adapt.
1. Purpose
- To confirm that each analytical balance used for GMP/testing is:
- Functioning correctly
- Within predefined accuracy/precision limits
- Safe to use for weighing of reagents, standards, and samples
If daily verification fails → do not use the balance for any GMP work until investigated and found acceptable.
2. What Needs to Be Defined in SOP
Your “Daily Balance Verification” SOP should clearly define:
- Scope
- All analytical balances used in:
- QC lab
- Production (dispensing, weighing rooms)
- R&D (if data used for CGMP decisions)
- All analytical balances used in:
- Frequency
- At the beginning of each day of use
- Additionally:
- After relocation
- After power failure
- If the balance shows abnormal behavior (drift, error messages, or suspected damage)
- Responsibility
- Trained analysts / production operators
- Review by QA/QC supervisor (for trend and OOS actions)
3. Pre-Checks Before Verification
Before starting daily checks:
- Ensure balance is level (spirit bubble centered).
- Draft shield doors closed (if applicable).
- Balance is clean (no spills or powder).
- Warm-up time completed (as per manufacturer).
- No strong air draft, vibration, or direct sunlight.
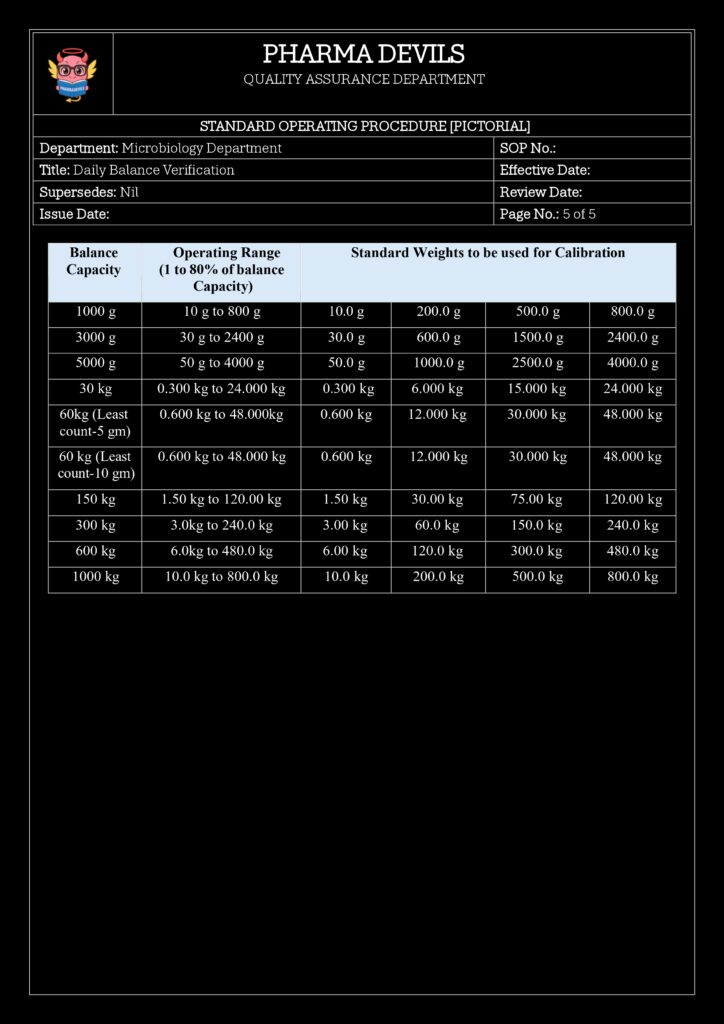
4. Standard Weights and Their Control
Use certified reference weights with known values and tolerances:
- Typically Class E2 / F1 OIML or equivalent, depending on balance readability.
- Maintain:
- Weight ID (e.g., W-10g-01)
- Nominal value (e.g., 10 g)
- Certificate number, traceability to national/international standards
- Calibration due date
Weights must be:
- Stored in clean, closed boxes.
- Handled with tweezers or gloves to avoid contamination.
- Kept near the balance (same environment) for temperature equilibrium.
5. Typical Daily Verification Procedure
You can express this as a step-wise checklist in the SOP/poster:
- Power On & Warm-Up
- Switch on balance (if not 24×7).
- Allow recommended warm-up period.
- Zero / Tare Check
- With empty pan, press ZERO.
- Confirm reading is 0.0000 g (or 0.00000 g depending on resolution).
- Internal Adjustment (If Available)
- Run internal calibration/adjustment as per manufacturer’s instructions.
- Document performed, if your SOP requires.
- Place Certified Weight(s)
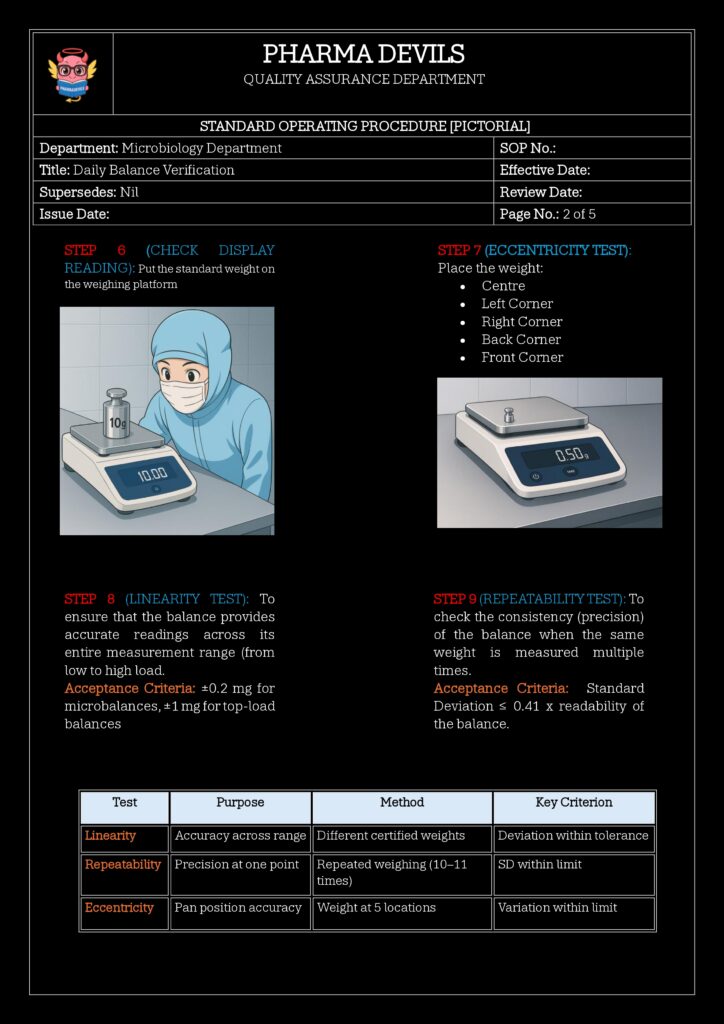
Recommended approach:- Use at least two weights:
- One near the lower or mid-range (e.g., 10 g)
- One near the upper range (e.g., 100 g) of routine use
- Record displayed value vs. nominal value.
- Use at least two weights:
- Calculate Error
- Error = |Displayed value – Nominal value|
- Check against predefined allowable tolerance (e.g., ±0.2 mg for 10 g, etc., based on risk and balance capability).
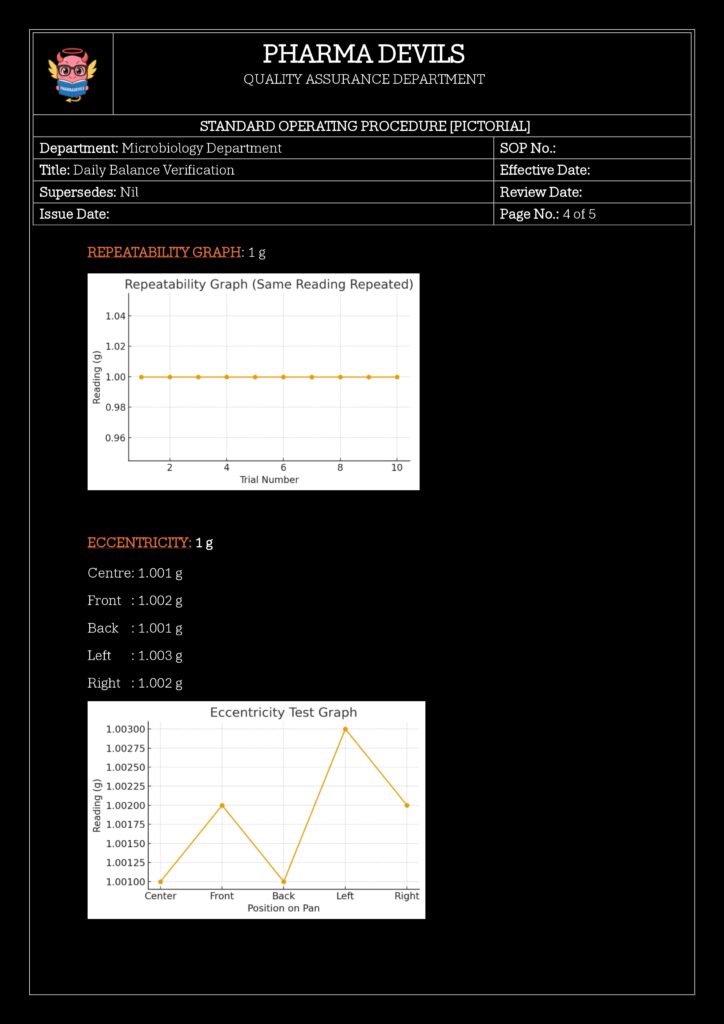
- Repeatability Check (Optional but Good Practice)
- Weigh the same weight 3–5 times, removing and replacing it.
- Calculate standard deviation (or range).
- Compare with defined acceptance criteria (e.g., σ ≤ 0.15 mg).
- Return to Zero
- Remove weight and ensure balance returns to 0.0000.
- If not, investigate and repeat as needed.
- Documentation
- Record in a log:
- Date & time
- Balance ID
- Weight ID(s)
- Nominal vs. observed values
- Calculated error
- Pass/Fail
- Analyst initials/signature
- Record in a log:
6. Acceptance Criteria (Example)
These must be defined in your validation/QRM, but common pattern:
- Accuracy:
- Error for 10 g weight must be within ±0.002 g
- Error for 100 g weight within ±0.02 g
(Adjust for your balance readability and process criticality.)
- Repeatability:
- Standard deviation ≤ 0.0002 g for 10 g weight over 5 measurements
- Or %RSD within predetermined limits
You can link these to your calibration certificate and balance qualification report.
7. What if the Balance Fails?
If any verification step fails:
- Immediately label the balance “OUT OF SERVICE”.
- Stop using it for any GMP weighing.
- Notify supervisor/QA.
- Initiate investigation:
- Check if wrong weight used, dirty pan, drafts, etc.
- Re-verify after corrective actions.
- Evaluate impact on previously weighed materials:
- Trace back to last successful verification.
- Assess impact on results and product quality.
- Document in deviation/OOS and decide on re-testing or re-weighing if possible.
8. Link to Calibration and Qualification
Daily verification is complementary to:
- Periodic external calibration (e.g., 6–12 months).
- Equipment qualification (IQ/OQ/PQ).
- Preventive maintenance.
Regulators expect:
- Documented evidence that equipment is fit for intended use at the time of use.
- Daily checks + calibration + qualification together provide that assurance.





