Risk-Based Inspection (RBI) – Schedule M Point 34.13 checks whether the Batch Packaging Record (BPR) covers all points prescribed in Schedule M. CDSCO Under RBI, packaging records are treated as high patient-risk because packaging errors can cause wrong label/strength, wrong batch/expiry, mix-ups, and recall events, even if manufacturing was correct.
What “BPR covers all points” means (Schedule M expectations)
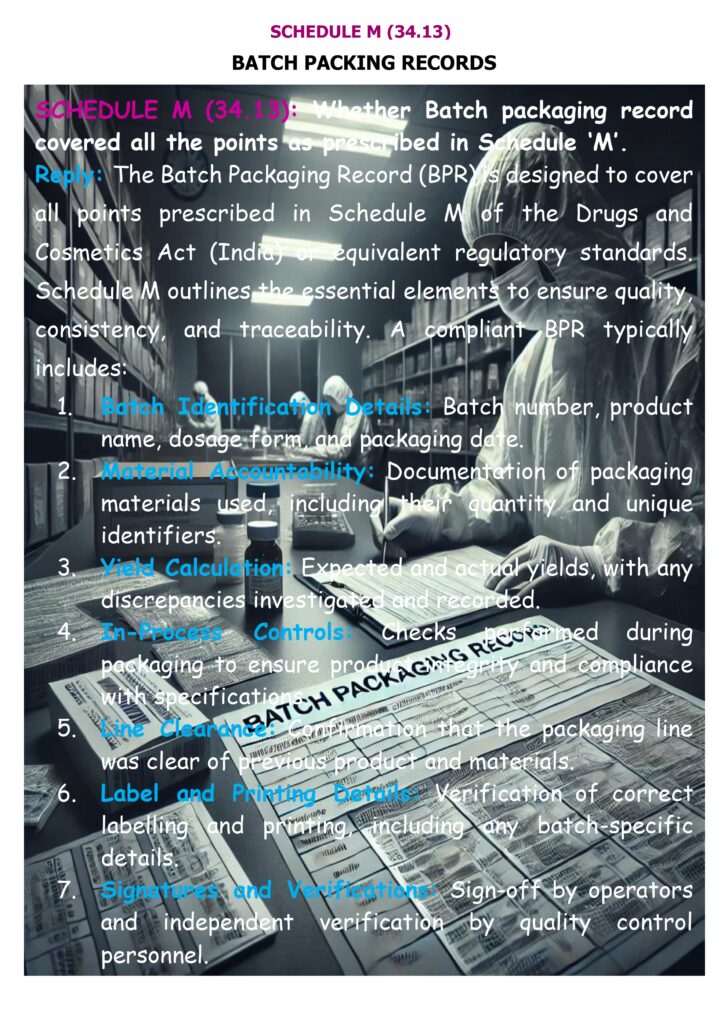
A compliant BPR is not just a filled form—it must demonstrate end-to-end control of the packaging operation. Key Schedule M requirements that must be reflected (directly or by referenced attachments) include:
- BPR exists for each batch/part-batch and is based on the approved packaging instructions, designed to avoid transcription errors.
- Pre-packaging line clearance checks are performed and recorded: before packaging begins, documented confirmation that equipment/workstation are clear of previous products, documents, and materials not required, and that equipment is clean and suitable.
- Packaging instructions coverage within the record set: product/strength, pack size, complete list of packaging materials with codes, specimens of printed components showing where batch/expiry are applied, special precautions (including careful examination for line clearance), packaging operations/equipment used, and in-process controls with sampling/acceptance limits.
- Reconciliation requirement: documented reconciliation of printed/issued packaging materials versus used, returned, and destroyed, with investigation of significant discrepancies before batch release.
How RBI inspectors verify 34.13 (practical sampling)
Inspectors typically select high-risk SKUs (look-alike packs, multiple strengths, recent artwork changes, high-volume products) and test whether the BPR package is complete, traceable, and reviewable, including:
- line clearance checklist(s) and signatures/time stamps,
- issuance and reconciliation sheets for labels/foils/cartons/leaflets,
- coding/overprint checks (batch/expiry correctness),
- in-process packaging checks and results,
- deviation/investigation/CAPA records linked to the batch,
- QA/IPQA review and release decision evidence.
Common RBI gaps: missing line-clearance records, weak reconciliation, uncontrolled attachments, or BPR content not matching current approved packaging instructions—these usually trigger deeper packaging-system review under RBI.





