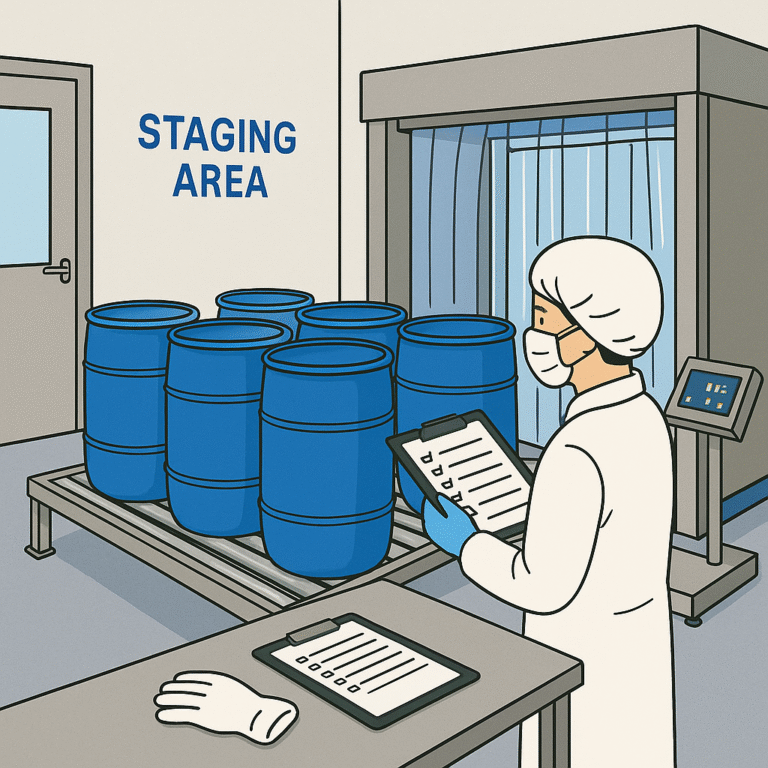
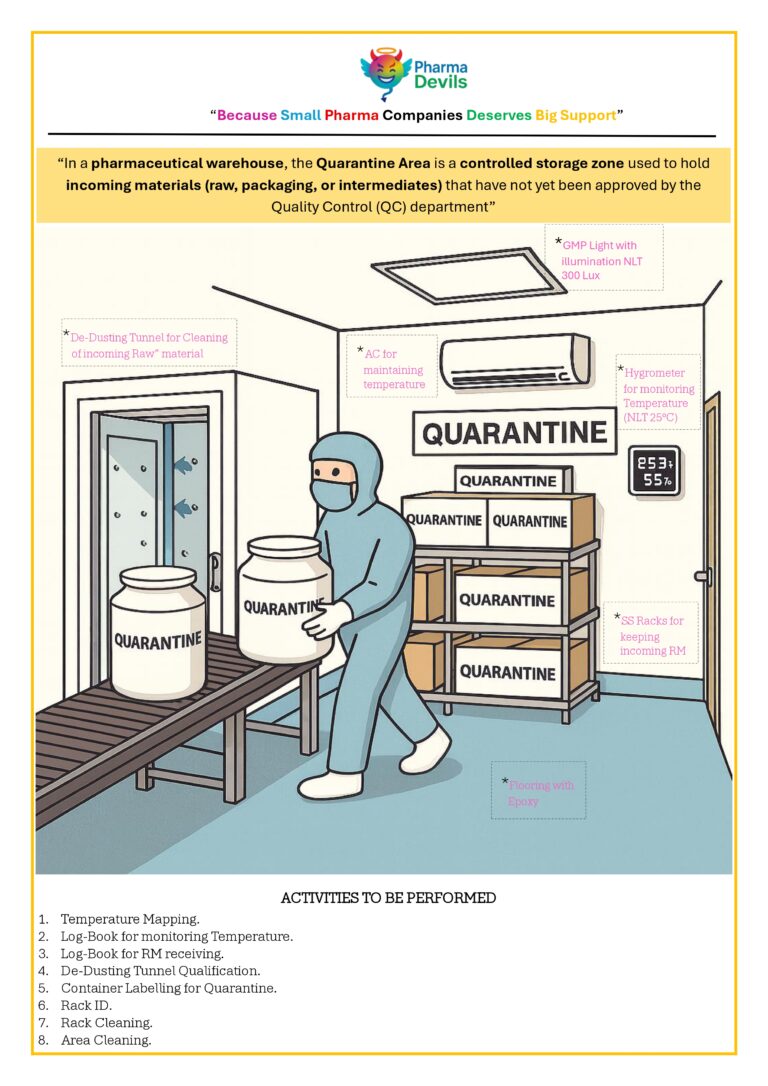
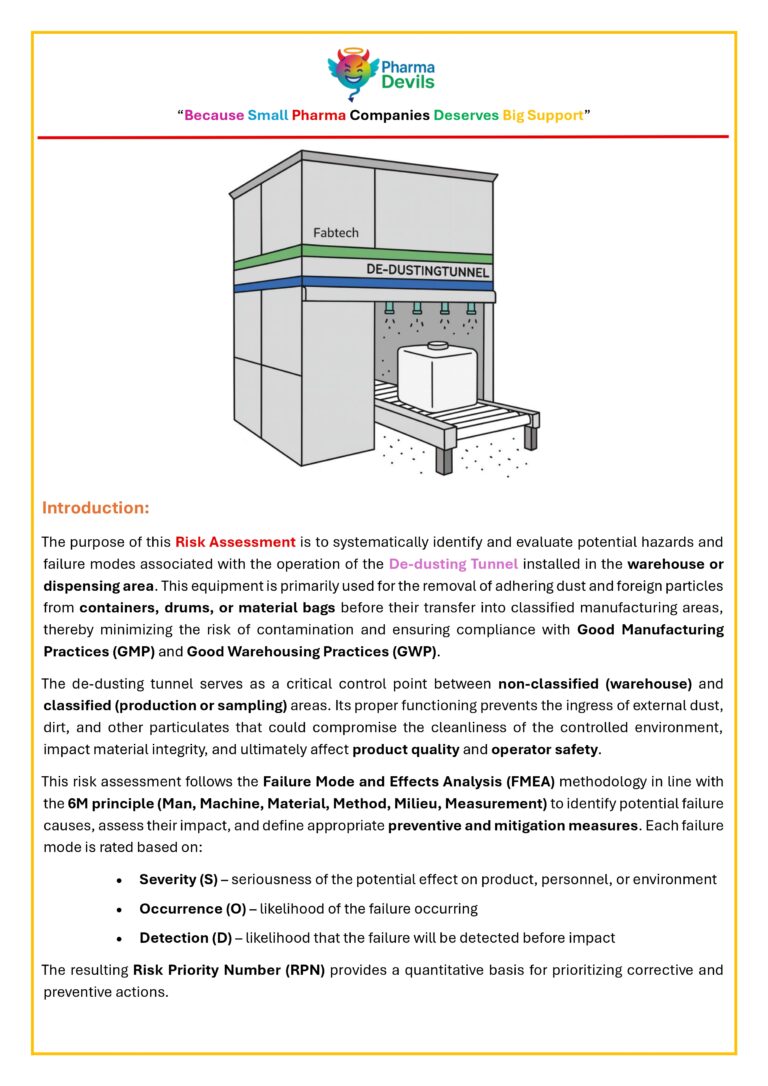
DECODING SOLVENT DISPENSING AREA IN PHARMA (PICTORIAL)
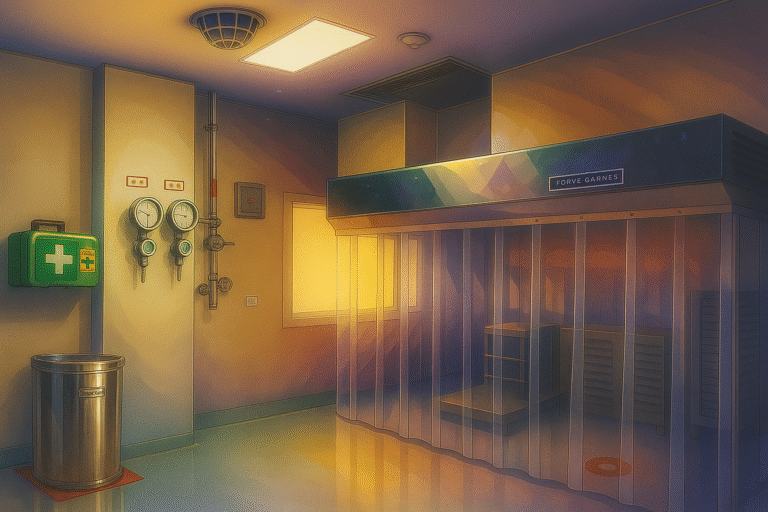
The Solvent Dispensing Area (SDA) is a controlled and ventilated workspace designed specifically for: Key Design Elements: Element Purpose Dispensing booth / laminar flow hood Provides local exhaust ventilation to capture solvent vapors. Often equipped with flameproof lighting and explosion-proof…