DRY POWDER INJECTION TESTS & CHECKS IN PHARMA
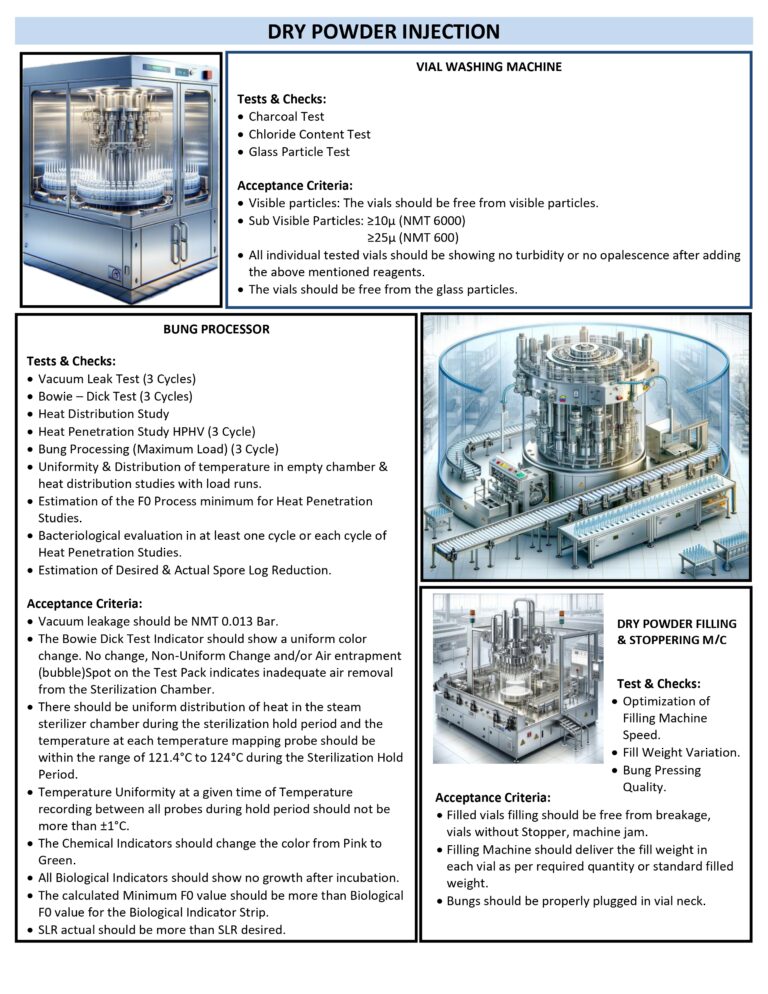
Dry Powder Injection (DPI) products (e.g., sterile powders in vials for reconstitution) require controls for sterility assurance, dose accuracy, powder performance, and container–closure integrity. Typical tests & checks span raw materials, in-process controls, and finished product testing. Incoming / raw…