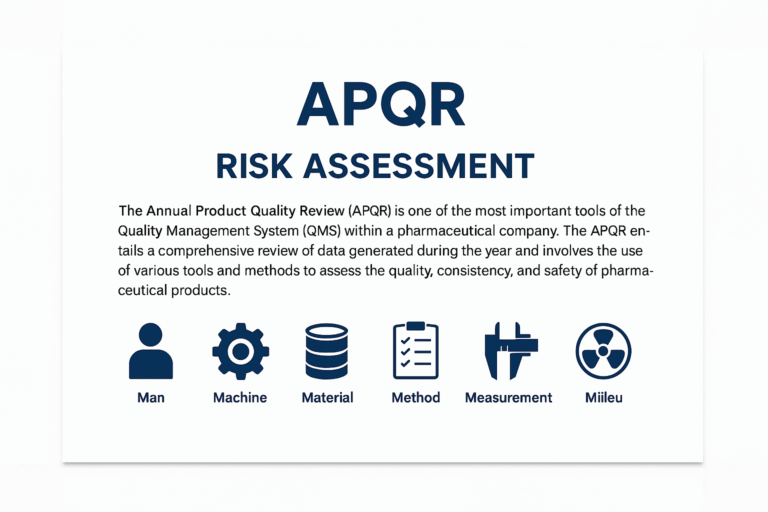
Risk Assessment for Analysis not done for Primary Packing Material

Introduction In pharmaceutical manufacturing, primary packaging materials are those that come into direct contact with the drug product, such as bottles, blister foils, tubes, vials, caps, stoppers, and laminates. These materials play a crucial role in protecting the product from…